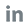Recently, Wondfo again received IFCC and NGSP certificates for Finecare™ HbA1c Assay with four instruments as we did in the past years. The certification verifies the reliable performance of Finecare™ FIA Meters in quantitative detection of hemoglobin A1c and trustworthy consistency across different laboratories. With repeated performance recognition from essays and authoritative verifications, Finecare™ FIA Meters are designed to assist in healthcare through convenient, rapid testing of sound quality.

IFCC (International Federation of Clinical Chemistry and Laboratory Medicine) and NGSP (National Glycohemoglobin Standardization Program) are organizations that establish and maintain standardized methods for measuring and reporting blood glucose levels. The standardized system for measuring HbA1c developed by them makes it possible to ensure consistent and reliable measurement of HbA1c across different laboratories and testing methods, which is critical for clinical healthcare providers to make decision and to provide proper treatment to patients with diabetes.
In April and May, Wondfo received multiple certificates granted by IFCC and NGSP respectively for HbA1c assay based on four Finecare™ FIA Meters (FS-112/113/114/205). These certificates verify the traceability of Finecare HbA1c rapid quantitative assay and demonstrate that Wondfo has been relentless in pursuing better outcomes of laboratory detection with constantly improved cutting-edge technology.

Since the launch of Finecare™ FIA Meters in 2009, the system has been widely distributed in 100+ countries and regions and highly appreciated by many users. Finecare™ System is easy to operate, rapid in delivering results (in 15 mins), capable of testing different items simultaneously and equipped with a comprehensive testing panel allowing various sample types (whole blood/serum/plasma). Up to now, more than 70 thousand units of Finecare™ FIA Meters have been installed in over 100 countries and regions worldwide, serving laboratories, ERs, ICUs, Cardiology Depts, etc.
Besides IFCC and NGSP verification, Finecare™ HbA1c assay has obtained various recognition such as essays and authoritative assessments. On March 23, 2023,
an evaluation of fluorescence immunoassay in quantitative measuring of HbA1c was published on Frontiers in Bioscience-Landmark (FBL), verifying that Wondfo’s Finecare™ FIA HbA1c Test is a reliable and rapid assay which can be easily implemented for long-term monitoring of HbA1c in diabetic patients. Furthermore,
in December 2022, the results of the External Quality Assurance Schemes for Reference Laboratories (EQAS for RELA)were released, including hemoglobin a1c and 16 other items of Wondfo Reference Laboratory.
Based on Wondfo’s multiple technology platforms and products lines, Wondfo is devoted to provide reliable and convenient testing for acute and critical cases, inflammation, coagulation and many others in various scenarios such as primary laboratory, chest pain center, pharmacy, etc.
Dedicated to make biology technology benefit the public, Wondfo strives for excellence with continuous efforts for the better, so as to improve the efficiency of diagnosis in medical institutions and to safeguard people’s health and happiness.
 The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc.
The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc. 50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others.
50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others. Single-dose Chemiluminescense Immunoassay Platform
Single-dose Chemiluminescense Immunoassay Platform Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.
Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously. Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.
Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals. Ready-to-use lyophilized RT-PCR Reagent;
Ready-to-use lyophilized RT-PCR Reagent; Wondfo PA-3600 IHC Staining System
Wondfo PA-3600 IHC Staining System The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds.
The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds. This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.
This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.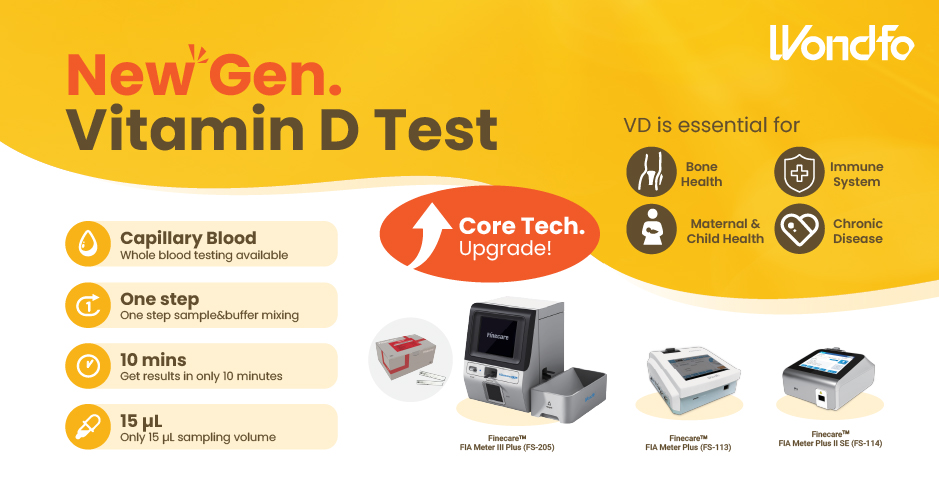 Finecare™ Vitamin D, from complexity to simplicity
Finecare™ Vitamin D, from complexity to simplicity Building A World Free from Antibitoic Overuse
Building A World Free from Antibitoic Overuse Advanced rapid diagnostic test with WHO prequalification for infectious disease
Advanced rapid diagnostic test with WHO prequalification for infectious disease The Future Intelligent Medical Assistant to Healthcare
The Future Intelligent Medical Assistant to Healthcare Fight against the pandemic through continuous innovation
Fight against the pandemic through continuous innovation