On the evening of April 25th, Wondfo Biotech (300482.SZ) reported first quarter 2023 results. The company achieved operating revenue of 832 million yuan, net profit attributable to the parent company of 204 million yuan, and net profit attributable to parent after deduction of non-recurring profit or loss of 195 million yuan. With the end of COVID-19 quarantine in 2022, the revenue of COVID-19 antigen test has dropped significantly. But the company’s regular business has returned normal under the revival of healthcare industry. Wondfo actively taps the full potential of the existing market and explores new markets, achieving rapid growth in conventional business compared to the same period last year.
Compared to the 1
st quarter before the pandemic, Wondfo’s operating revenue in the first quarter of 2023 showed a leap in growth, with an increase of 83.79% compared to the same period in 2019. The gross profit margin reached 65.30%, 3.75 percentage points higher than the same period in 2019, indicating a strong development momentum.
From the perspective of business lines, in terms of infectious disease detection, the H1N1 outbreak in the first quarter drove the sales of influenza testing reagents for the company, resulting in a significant growth in regular infectious disease testing business that exceeded expectations. In terms of chronic disease management business, the volume of outpatients and samples of domestic high-level hospitals and primary medical institutions recovered to varying degrees in the first quarter. Auxiliary indicators for diagnosis and treatment of COVID-19 also drove the operating performance of regular items of the quantitative detection platforms, driving the business of chronic disease management solution to grow significantly. At the same time, the fertility testing business and DOA testing business continue to maintain steady growth.
From the prospective of product lines, Wondfo will witness a climax of return on new products in 2023. The company’s immunofluorescence platform is undergoing comprehensive technological upgrades, and there are plans for registration of several items from the chemiluminescence immunoassay platform. The new products of molecular diagnosis and pathological diagnosis platforms are experiencing accelerated product registration process. The constant launch and registration of new products is believed to effectively drive operating revenue and sound profit, contributing new chances of business growth.
In terms of overseas business, Wondfo will strengthen the localized operation capacity this year in key countries overseas, introduce new main platforms while stabilizing the basic businesses, and actively facilitate the quadratic growth curve regarding our business. Based on the first-mover advantage in the international market and the large-scale instrument inventory, we will continue to improve our distribution coverage overseas and value created by instruments, promoting the sustained and stable development of overseas business.
2023 is a year of readjustment and renewal for the in vitro diagnostic industry. In this changing situation, Wondfo will balance short-term and long-term goals, orderly promote the implementation of strategic goals, and continue to consolidate our product and market advantages. At the same time, we will promote cost reduction and efficiency increase, improve business quality, and pursue a path of high-quality development, striving to become an IVD enterprise with global influence.
 The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc.
The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc. 50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others.
50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others. Single-dose Chemiluminescense Immunoassay Platform
Single-dose Chemiluminescense Immunoassay Platform Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.
Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.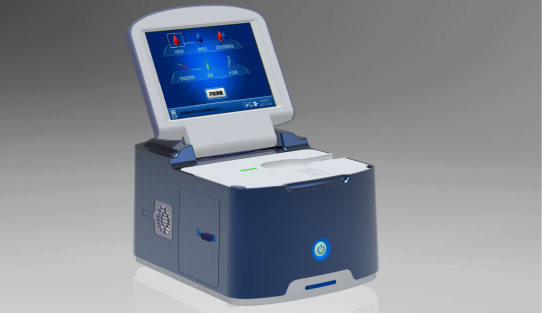 Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.
Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals. Ready-to-use lyophilized RT-PCR Reagent;
Ready-to-use lyophilized RT-PCR Reagent; Wondfo PA-3600 IHC Staining System
Wondfo PA-3600 IHC Staining System The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds.
The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds. This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.
This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.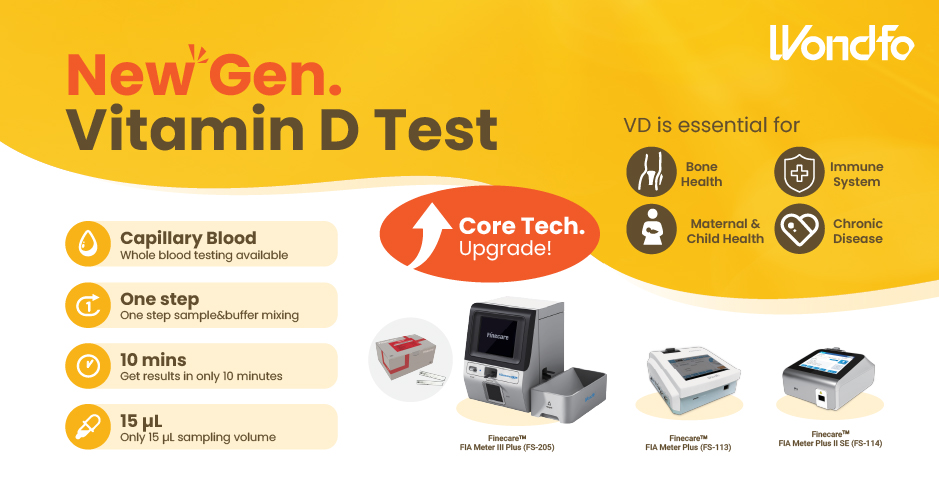 Finecare™ Vitamin D, from complexity to simplicity
Finecare™ Vitamin D, from complexity to simplicity Building A World Free from Antibitoic Overuse
Building A World Free from Antibitoic Overuse Advanced rapid diagnostic test with WHO prequalification for infectious disease
Advanced rapid diagnostic test with WHO prequalification for infectious disease The Future Intelligent Medical Assistant to Healthcare
The Future Intelligent Medical Assistant to Healthcare Fight against the pandemic through continuous innovation
Fight against the pandemic through continuous innovation