1. Introduction
Prostate cancer (PC) originates in the prostate gland, a male sex gland responsible for semen production, located below the bladder and in front of the rectum. As per the 2022 Global Cancer Observatory report, it is the fourth most common cancer globally, with 1,467,854 cases and 397,430 deaths, ranking eighth in cancer-related mortality [5]. While most cases occur in developed countries, Africa records the highest mortality rate. Early diagnosis is critical, as prostate cancer is highly treatable when detected early, significantly improving patient survival outcomes [1].
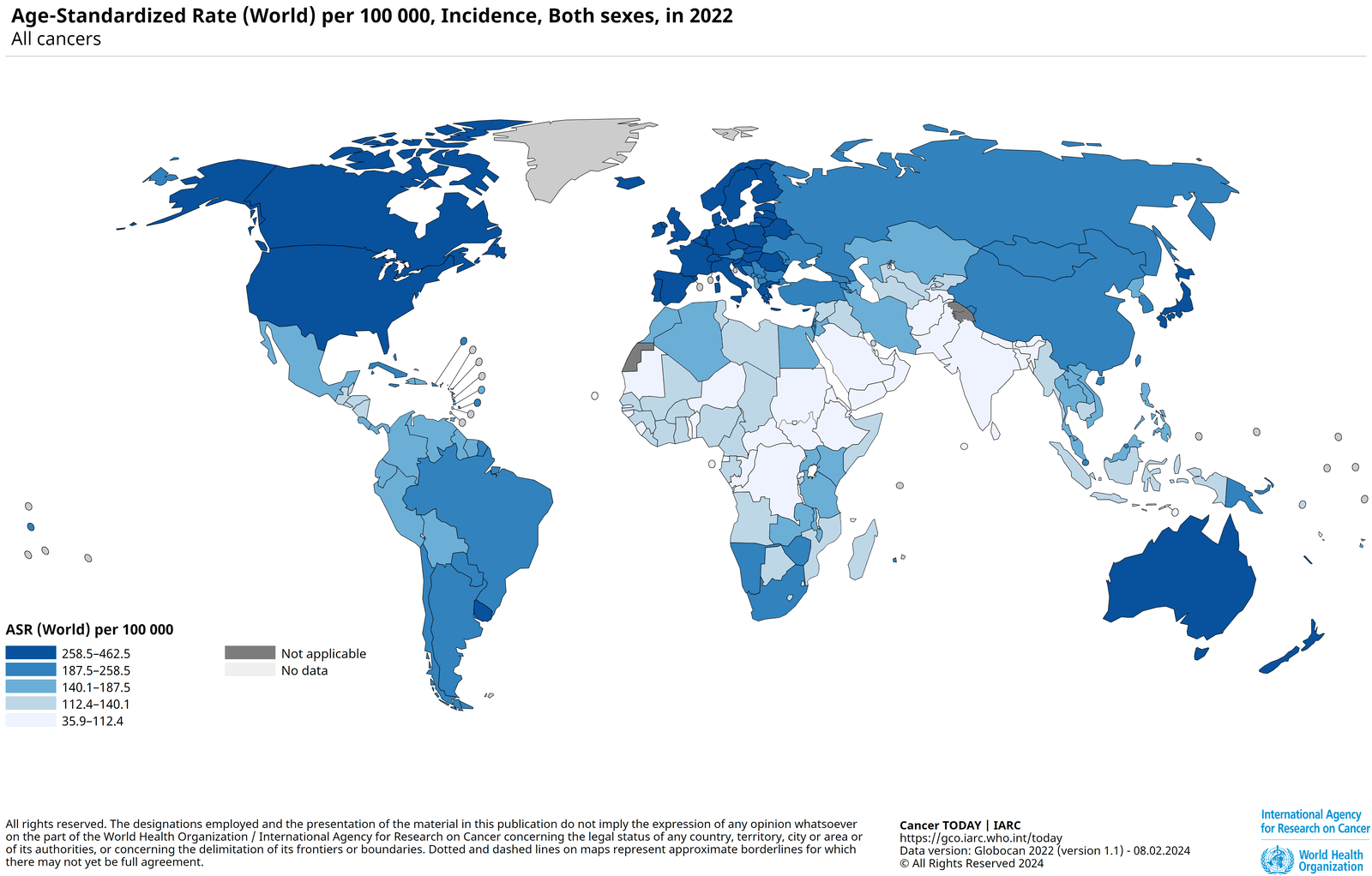
Fig 1: Incidence of Prostate Cancer, ASR (World) per 100,000 [5]
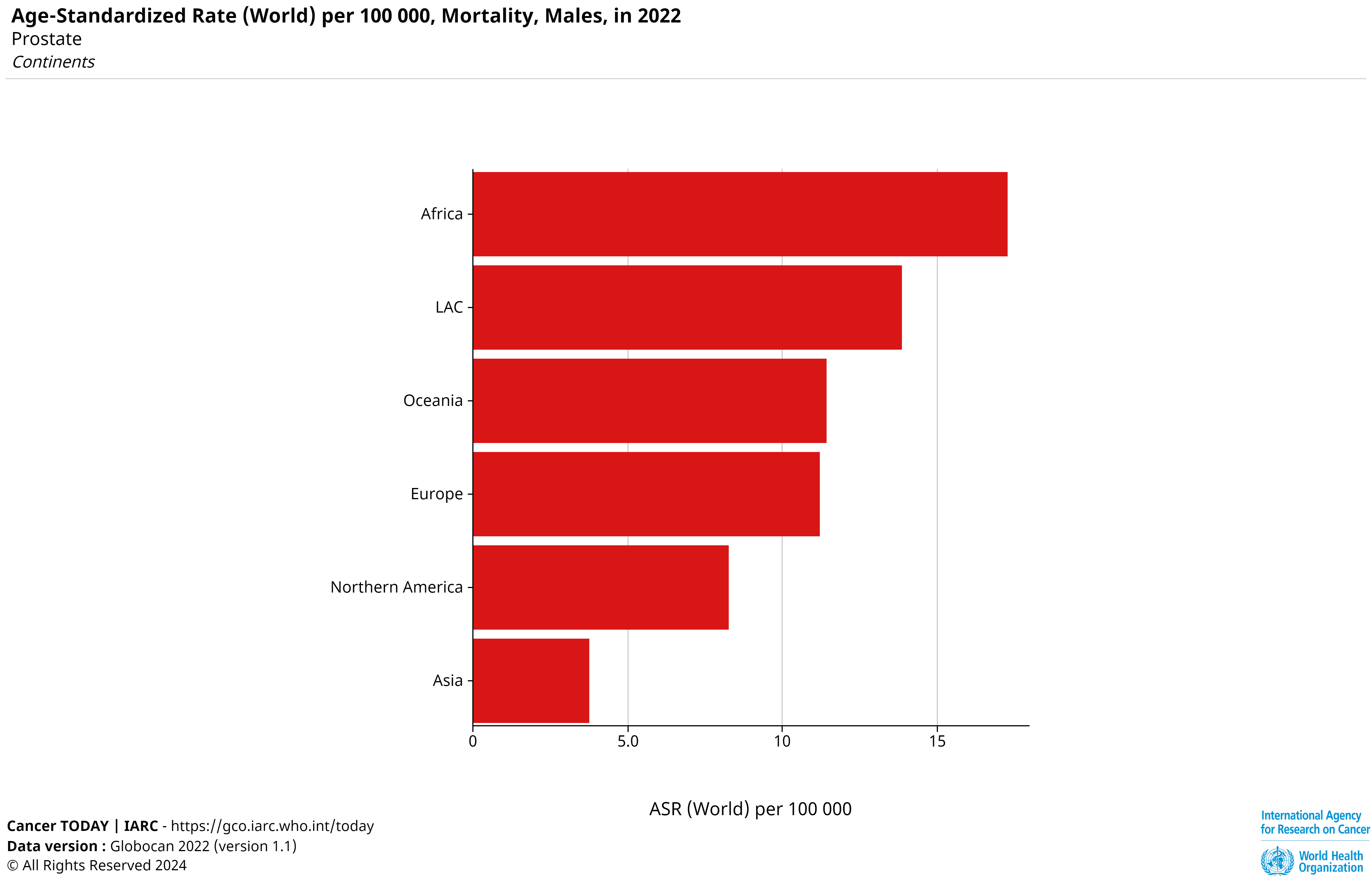
Fig 2: Mortality of Prostate Cancer, ASR (World) per 100,000 [5]
Prostate-Specific Antigen (PSA) is a protein produced by the prostate gland, predominantly present in semen but also measurable in the bloodstream. Elevated serum PSA levels, detected through blood testing, serve as a key biomarker for prostate health, potentially indicating conditions such as prostate cancer, benign prostatic hyperplasia (BPH), or prostatitis [2]. In cases of prostate cancer, epithelial cells produce excess PSA, leading to heightened serum levels. Within the bloodstream, PSA exists in both free and bound forms, with the latter involving binding to serine protease inhibitors [4].
2. PSA Levels Aiding in Screening of Prostate Cancer
2.1 PSA in Early Screening
PSA testing is a key tool for early prostate cancer screening, particularly for individuals with risk factors such as age over 50, African ancestry, family history, or genetic predispositions like BRCA2 mutations or Lynch syndrome [2]. These factors guide screening timelines, with some men starting as early as 40 or 45 years [1].
Normal PSA levels are below 4 ng/mL, though 15% of prostate cancer cases occur within this range. Levels between 4 and 10 ng/mL indicate a 25% likelihood of cancer, while levels above 10 ng/mL suggest a risk exceeding 50%. Age-specific cutoffs or lower thresholds (e.g., ≥3 ng/mL) may improve sensitivity [2].
Elevated PSA levels should be confirmed through repeat testing due to potential influences like infections or 5-alpha-reductase inhibitors. Persistent elevation warrants further evaluation with imaging (e.g., TRUS) and biopsy, which provides definitive diagnosis and guides treatment. PSA interpretation must consider risk factors for optimal early detection and outcomes [1].
2.2 Early Diagnosis to Improve Patient Outcomes
Screening for prostate cancer offers the significant benefit of detecting the disease at an earlier, more treatable stage. According to data from the SEER (Surveillance, Epidemiology, and End Results) database maintained by the National Cancer Institute, the 5-year relative survival rates for prostate cancer vary based on its spread. For localized and regional cases, where the cancer is confined to the prostate or nearby structures/lymph nodes, the survival rate is greater than 99%. However, for distant cases, where the cancer has metastasized to organs like the lungs, liver, or bones, the survival rate drops to 34%. Overall, the combined 5-year relative survival rate for all SEER stages is 97% [2]. These figures are based on diagnoses from 2013 to 2019.
3. Monitoring Prostate Cancer Progression
3.1. The Importance of Regular PSA Testing for Monitoring Disease Progression and Treatment Response
For men who have undergone radical prostatectomy (RP), PSA levels should be undetectable (typically <0.1 ng/mL) within two months. Biochemical recurrence (BCR) is defined by any rising PSA level after RP, with follow-up PSA tests typically done every six months for the first three years and annually thereafter [1]. Early recurrences may require more frequent testing, while those with a life expectancy under 10 years may have follow-up discontinued. A rise of 2 ng/mL above the nadir or three consecutive PSA increases within six months are indicators of recurrence. A PSA nadir below 0.01 ng/mL predicts a 96% chance of remaining relapse-free within two years [1]. Post-treatment PSA patterns vary: after surgery, levels should drop to undetectable, while after radiotherapy, a temporary rise (PSA bounce) may occur [2]. PSA levels rising above 0.2 ng/mL are considered indicative of treatment failure, whereas stable levels between 0.2 and 1.0 ng/mL may not necessarily imply the same. [6]. If PSA levels rise gradually after treatment, it may still be considered for further assessment, particularly in cases where the neurovascular bundle is reserved during surgery, and repeat treatments may be indicated [4].
3.2. Implications of Fluctuating PSA Levels Over Time
Research indicates that patients with fluctuating PSA levels after treatment often have better survival outcomes than those with continuous increases. A study showed that patients with fluctuating PSA had a median overall survival of 18 months, compared to 8 months for those with continuous increases [6]. This suggests that transient fluctuations should not immediately lead to treatment discontinuation. Management of fluctuating PSA levels requires careful monitoring, considering factors like the absence of radiological progression, before deciding on interventions such as biopsies or treatment adjustments. This approach allows for a more refined understanding of the patient's condition and avoids unnecessary treatment changes.
4. Conclusion
In conclusion, serum PSA testing is essential for the early detection and ongoing monitoring of prostate cancer, significantly improving survival rates, particularly when the disease is identified at localized stages. Regular post-treatment PSA monitoring aids in detecting recurrence, with fluctuating levels often requiring careful interpretation rather than immediate intervention. The utility of PSA testing has been greatly enhanced by advanced diagnostic technologies such as Fluorescent Immunoassay (FIA) and Chemiluminescence Immunoassay (CLIA). FIA provides high sensitivity, precision, and rapid results by using fluorophores, while CLIA, employing luminescent molecules, offers superior stability, signal intensity, and broad dynamic range, making it suitable for high-throughput environments [4]. Wondfo further elevates prostate cancer diagnostics by offering comprehensive PSA marker panels compatible with both FIA and CLIA platforms, providing adaptable solutions for diverse clinical scenarios, including general practice offices, screening programs, cancer associations, and oncology wards. This integrated approach underscores the critical role of PSA testing in optimizing patient outcomes and advancing prostate cancer care.

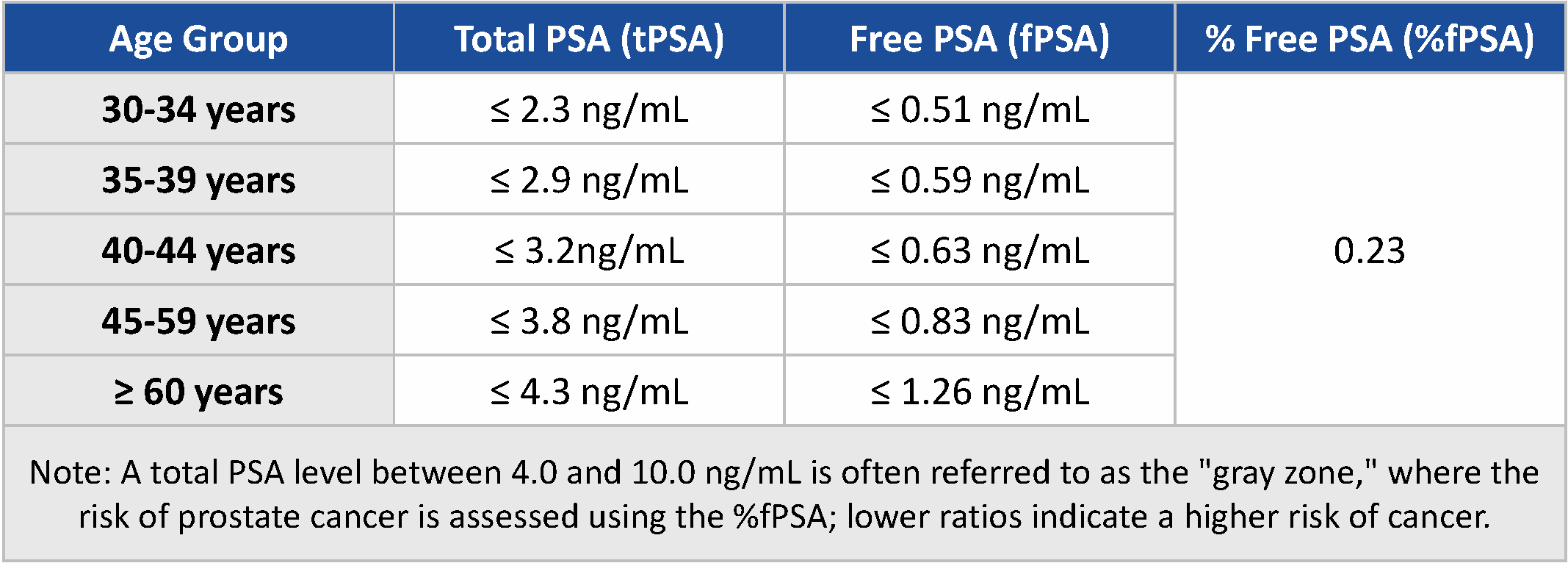
Fig 3: Age-Specific Reference Range for Serum Level of tPSA, fPSA, and fPSA-tPSA Ratio [7]
References:
1. European Association of Urology (EAU). (2024). Prostate Cancer Guidelines. Retrieved November 21, 2024 from https://uroweb.org/guidelines/prostate-cancer
2. American Cancer Society. (n.d.). What Is Prostate Cancer? Retrieved November 21, 2024, from https://www.cancer.org/cancer/types/prostate-cancer/about/what-is-prostate-cancer.html
3. Morita, M., & Matsuura, T. (2012). Management of localized prostate cancer by focal transurethral resection of prostate cancer: An application of radical TUR-PCa to focal therapy. Advances in Urology, 2012, Article ID 564372. https://doi.org/10.1155/2012/564372
4. Khan, R., Arshad, F., Hassan, I. U., Naikoo, G. A., Pedram, M. Z., Saeedi Zedegan, M., Pourfarzad, H., Aljabali, A. A. A., Serrano-Aroca, Á., Haggag, Y., Mishra, V., Mishra, Y., Birkett, M., Tambuwala, M. M. (2022). Advances in nanomaterial-based immunosensors for prostate cancer screening. Biomedicine & Pharmacotherapy, 155, 113649. https://doi.org/10.1016/j.biopha.2022.113649
5. Ferlay, J., Ervik, M., Lam, F., Laversanne, M., Colombet, M., Mery, L., Piñeros, M., Znaor, A., Soerjomataram, I., & Bray, F. (2024). Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. Retrieved November 21, 2024 from https://gco.iarc.who.int/today
6. Hartrampf, P. E., Bundschuh, R. A., Weinzierl, F.-X., Serfling, S. E., Kosmala, A., Seitz, A. K., Kübler, H., Buck, A. K., Essler, M., & Werner, R. A. (2022). mCRPC patients with PSA fluctuations under radioligand therapy have comparable survival benefits relative to patients with sustained PSA decrease. European Journal of Nuclear Medicine and Molecular Imaging, 49(12), 4727-4735. https://doi.org/10.1007/s00259-022-05910-w
7. Battikhi, M. N. G. (2003). Age-specific reference ranges for prostate-specific antigen (PSA) in Jordanian patients. Prostate Cancer and Prostatic Diseases, 6, 256-260. https://doi.org/10.1038/sj.pcan.4500656
 The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc.
The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc. 50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others.
50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others. Single-dose Chemiluminescense Immunoassay Platform
Single-dose Chemiluminescense Immunoassay Platform Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.
Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously. Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.
Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals. Ready-to-use lyophilized RT-PCR Reagent;
Ready-to-use lyophilized RT-PCR Reagent; Wondfo PA-3600 IHC Staining System
Wondfo PA-3600 IHC Staining System The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds.
The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds. This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.
This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers. Finecare™ Vitamin D, from complexity to simplicity
Finecare™ Vitamin D, from complexity to simplicity Building A World Free from Antibitoic Overuse
Building A World Free from Antibitoic Overuse Advanced rapid diagnostic test with WHO prequalification for infectious disease
Advanced rapid diagnostic test with WHO prequalification for infectious disease The Future Intelligent Medical Assistant to Healthcare
The Future Intelligent Medical Assistant to Healthcare Fight against the pandemic through continuous innovation
Fight against the pandemic through continuous innovation