1. Introduction
Type II diabetes is a global health challenge that affects over 422 million people worldwide [2]. Early detection and effective monitoring are critical for managing this chronic condition, as they can significantly improve patient outcomes by reducing the risk of complications such as cardiovascular disease, kidney failure, and blindness. In response to this need, diagnostic techniques have evolved, offering both Point-of-Care Testing (POCT) devices and laboratory-based methods like High-Performance Liquid Chromatography (HPLC). These technologies play an indispensable role in the accurate measurement of glycated hemoglobin (HbA1c), which is key to assessing long-term glucose control.
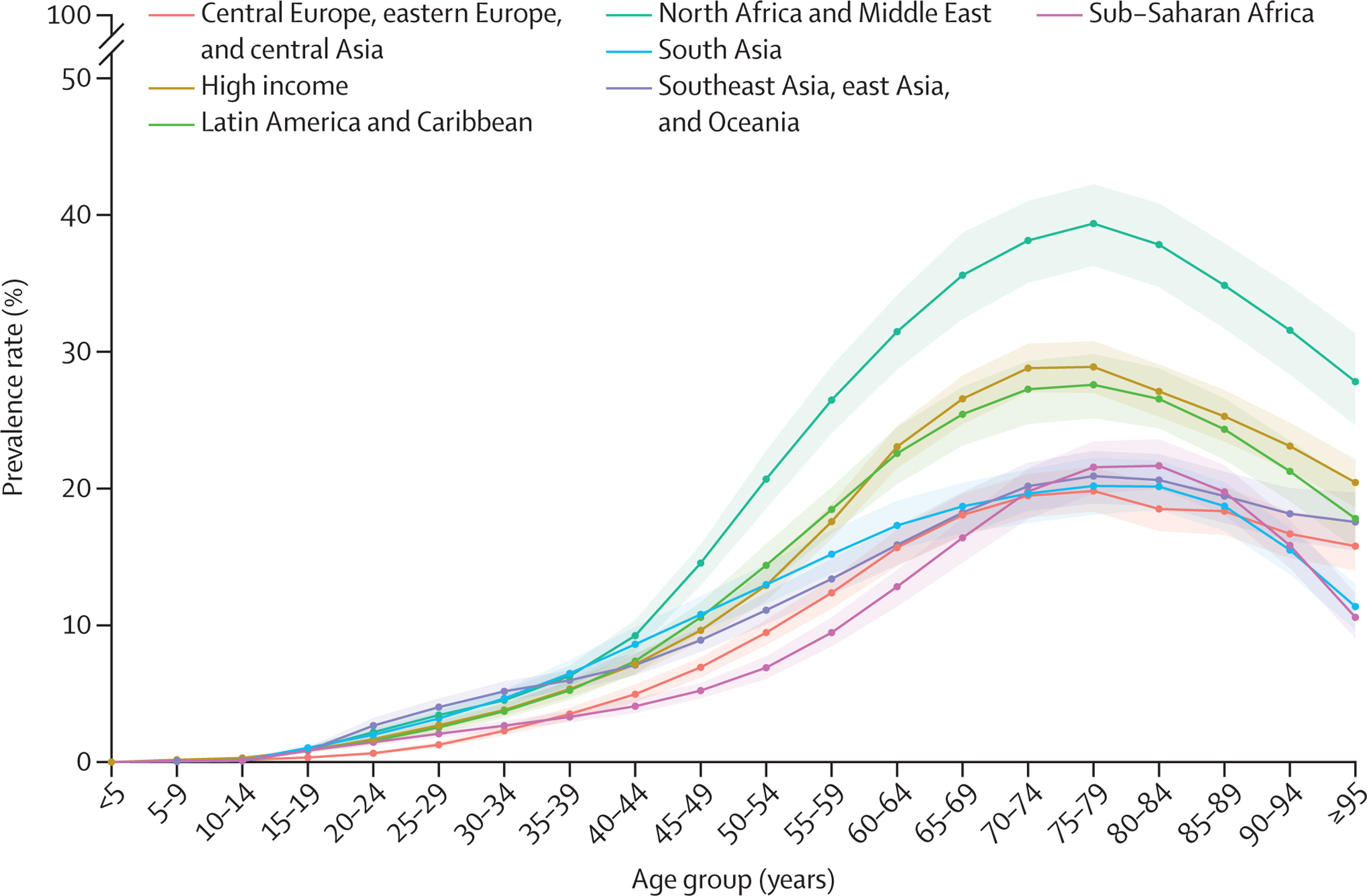
Fig 1: Prevalence of total diabetes by age and Global Burden of Diseases super-region in 2021 adopted from [1].
2. POCT Immunofluorescent Assays: A Game-Changer in Diabetes Management
Immunofluorescent assays represent a breakthrough in rapid diagnostics for HbA1c measurement. This technology utilizes antibodies specific to HbA1c, which bind to the molecule and emit fluorescence when excited by light. The intensity of the emitted light correlates with the concentration of HbA1c in the sample [3]. POCT immunoassays offer several advantages:
-
Enhanced Clinical Outcomes: Immediate results enable timely treatment adjustments, improving glycemic control and reducing long-term complications. [6]
-
Cost-Effectiveness in Primary Care: While initial device costs exist, POCT demonstrates favorable cost-effectiveness through reduced hospitalization rates and diabetes-related complications. [6]
-
Improved Access: Suitable for decentralized testing in primary care settings and underserved areas, increasing testing frequency and early intervention opportunities.
-
Operational Efficiency: Minimal training requirements and rapid turnaround (<5 minutes) facilitate workflow integration in busy clinical environments.
Collectively, these advancements position POCT immunoassays as a clinically effective and operationally viable solution for HbA1c monitoring, demonstrating significant potential to improve diabetes management outcomes in diverse healthcare settings.
3. HPLC: The Gold Standard in Diabetes Diagnostics
HPLC remains the gold standard for HbA1c analysis, offering excellent precision in differentiating haemoglobin variants, including those associated with haemoglobinopathies [4]. By separating blood components via physicochemical properties, HPLC enables accurate HbA1c quantification. Key advantages include automated calibration, minimal carry-over via polished steel probes, and seamless data integration with bidirectional LIS systems. Enhanced column efficiency - achieved through sharp peak resolution, high-density gel, and user-replaceable columns - ensures consistent performance. Additionally, patented exhaust systems and side-hole needles reduce downtime and improve sampling accuracy. Study showed Elevated foetal haemoglobin (HbF) in some cases may interfere with results [5]; however, HPLC’s reliability and precision solidify its role in robust diabetes management, balancing analytical accuracy with practical adaptability.
4. Choosing the Right Tool
The choice between POCT immunofluorescent assays and HPLC should align with the clinical setting and the type of information needed:
-
POCT Immunofluorescent Assays: Suited for low-volume, decentralised settings (e.g., primary care, diabetes clinics, rural or resource-limited environments), POCT enables rapid screening, diagnosis, and routine patient follow-up, supporting timely clinical decision-making.
-
HPLC: Designed for high-throughput, centralised laboratories (e.g., secondary/tertiary hospitals), HPLC aids in diagnostic subtyping and longitudinal monitoring, offering precise analysis for complex cases.
These two methodologies complement each other, allowing healthcare providers to tailor their approach to the specific needs of patients and facilities.
5. Conclusion
In conclusion, both POCT immunoassays and HPLC play complementary roles in advancing Type II diabetes management. Modern POCT solutions deliver rapid, accessible HbA1c testing with proven reliability, empowering clinicians to make timely decisions in diverse settings. HPLC remains a cornerstone for centralized laboratories, offering high precision in complex diagnostic scenarios. By strategically integrating these technologies - healthcare systems can achieve comprehensive, patient-centric care while driving efficiencies across care pathways.
Wondfo’s Finecare™ fluorescence immunoassay platform provides rapid and reliable HbA1c testing, delivering results within 5 minutes. An evaluation by Qatar University demonstrated excellent agreement with a top-tier biochemistry-immunoassay system, Finecare™HbA1c test with fingerstick sample showing 100% sensitivity and 98.7% specificity with venipuncture samples [7]. Finecare™ also significantly differentiated between normal, pre-diabetic, and diabetic samples (p < 0.0001). This makes it an ideal tool for efficient long-term HbA1c monitoring in diabetic patients, supporting swift clinical decisions.

Fig 2: (1)Most popular model FS-114 from Finecare™ series; (2) Package of Finecare™ HbA1c reagents; (3) Pairwise correlation and linear regression analysis of Finecare™ Fingerstick Test in comparison to Cobas Pro c503 adopted from [7].
For more precise and comprehensive analysis, our advanced HPLC systems excel by identifying common glycosylated hemoglobin variants and simultaneously reporting IFCC and NGSP results. They accurately handle abnormal hemoglobin samples thanks to sharp peak resolution and high-density gel for enhanced column efficiency. Features such as a patented automatic exhaust system, side-hole needles for precise sampling, and easily replaceable chromatographic columns ensure optimal performance and reduced downtime. This combination of speed and precision makes our HPLC systems an indispensable tool for accurate diabetes diagnostics.
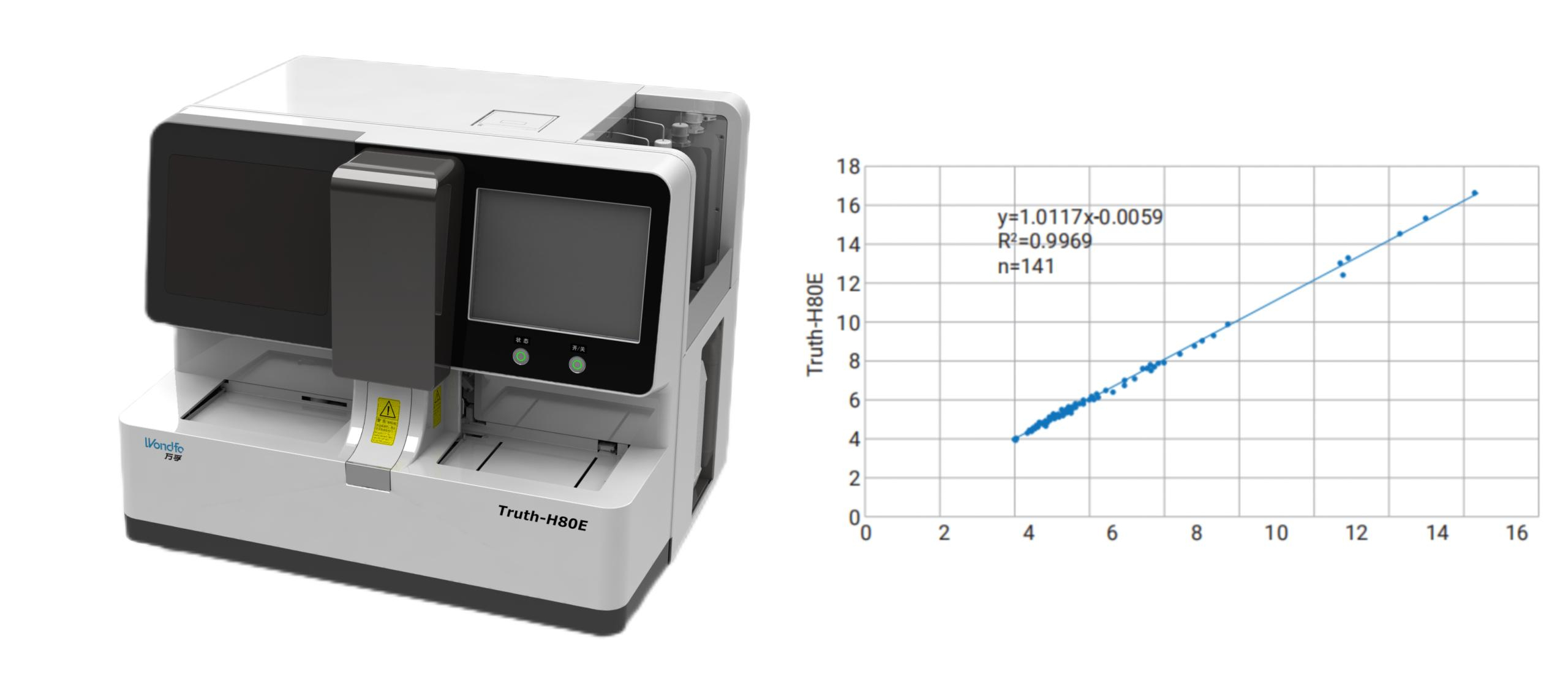
Fig 3: Image for Wondfo Truth-H80E (left) and correlation between Truth-H80E with mainstream models (right)
Healthcare providers are encouraged to consider the unique attributes of each method to select the most appropriate solution for their practice. By doing so, they can ensure timely and accurate monitoring of HbA1c levels, thereby enhancing the quality of care for individuals living with diabetes.
References:
1. GBD 2021 Diabetes Collaborators. (2023). Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. The Lancet, 402(10397), 203-234. https://doi.org/10.1016/S0140-6736(23)01301-6
2. World Health Organization. (2023). Diabetes. Retrieved from https://www.who.int/news-room/fact-sheets/detail/diabetes
3. Agrawal, S., Reinert, S. E., Baird, G. L., & Quintos, J. B. (2018). Comparing HbA1c by POC and HPLC. Rhode Island Medical Journal, 101(9), 44-46.
4. Hoelzel, W., Weykamp, C., Jeppsson, J., Kobold, U., Miedema, K., & Finke, A. (2004). IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method-comparison study. Clinical Chemistry, 50(1), 166-174.
5. National Glycohemoglobin Standardization Program. (n.d.). Factors that Interfere with HbA1c Test Results. Retrieved from https://ngsp.org/factors.asp
6. Rosa, L. S., Mistro, S., Oliveira, M. G., Kochergin, C. N., Cortes, M. L., Medeiros, D. S, ... & Passos, L. C. (2021). Cost-Effectiveness of Point-of-Care A1C Tests in a Primary Care Setting. Frontiers in Pharmacology, 11, 588309. https://doi.org/10.3389/fphar.2020.588309
7. Younes, N., Al Ghwairi, M. M., Da’as, S. I., Al Zaabi, E., Majdalawieh, A. F., Al-Dewik, N., & Nasrallah, G. K. (2023). Performance evaluation of a new fluorescent-based lateral flow immunoassay for quantification of hemoglobin A1c in diabetic patients. Original Research, 1-15. https://doi.org/10.31083/j.fbl2803060
 The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc.
The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc. 50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others.
50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others. Single-dose Chemiluminescense Immunoassay Platform
Single-dose Chemiluminescense Immunoassay Platform Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.
Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously. Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.
Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals. Ready-to-use lyophilized RT-PCR Reagent;
Ready-to-use lyophilized RT-PCR Reagent; Wondfo PA-3600 IHC Staining System
Wondfo PA-3600 IHC Staining System The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds.
The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds. This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.
This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers. Finecare™ Vitamin D, from complexity to simplicity
Finecare™ Vitamin D, from complexity to simplicity Building A World Free from Antibitoic Overuse
Building A World Free from Antibitoic Overuse Advanced rapid diagnostic test with WHO prequalification for infectious disease
Advanced rapid diagnostic test with WHO prequalification for infectious disease The Future Intelligent Medical Assistant to Healthcare
The Future Intelligent Medical Assistant to Healthcare Fight against the pandemic through continuous innovation
Fight against the pandemic through continuous innovation