1. Case Introduction:
Case Timeline
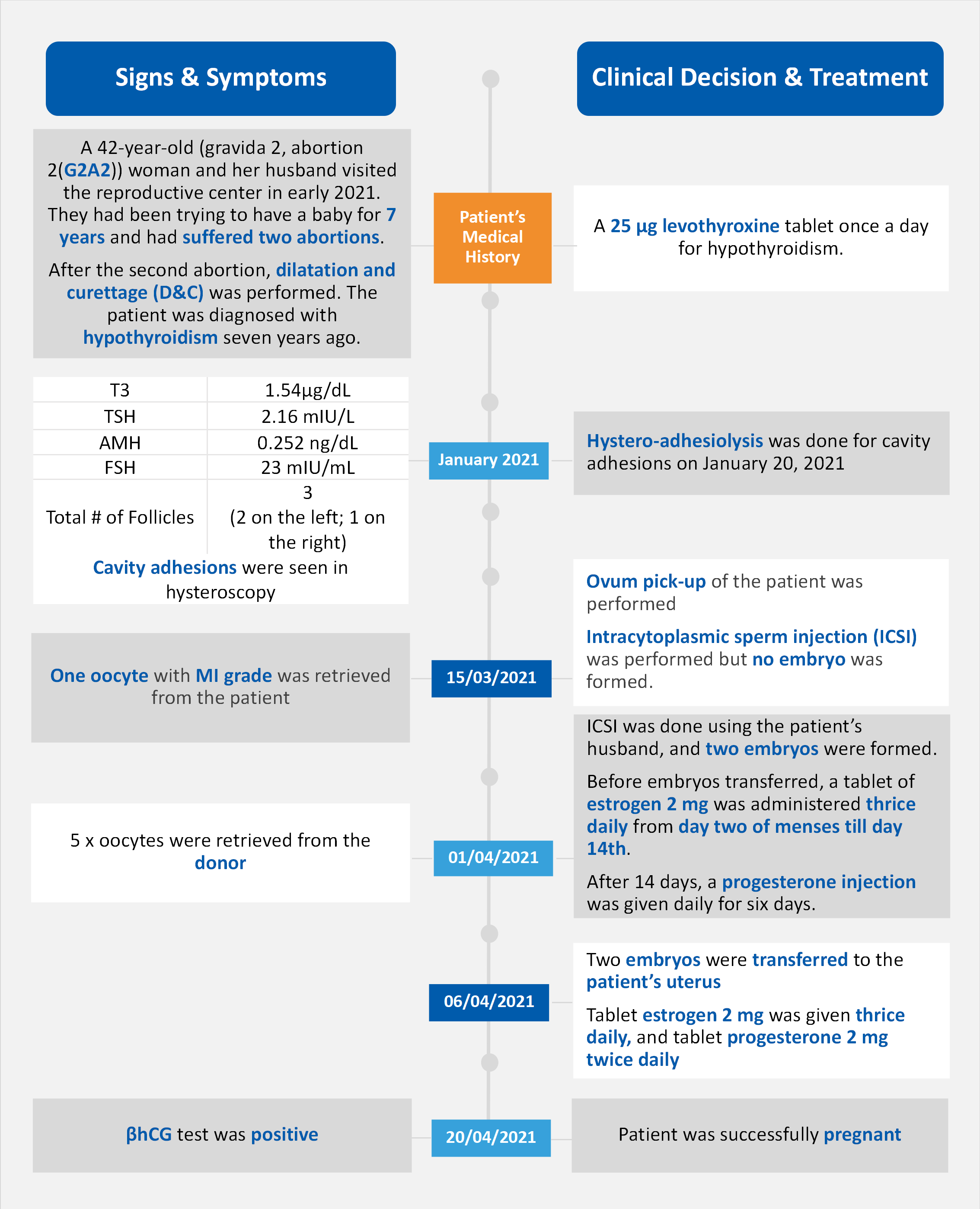
The 42-year-old patient initially consulted with a fertility clinic due to secondary infertility after two previous abortions. Her initial evaluation revealed elevated FSH levels (23 mIU/mL) and low AMH levels (0.252 ng/mL), indicating poor ovarian reserve [1]. According to the American Society for Reproductive Medicine (ASRM), these markers are strong indicators of diminished ovarian reserve (DOR) [4]. Two previous IVF cycles with self-oocytes resulted in poor ovarian response and no fertilization, further confirming her high risk of DOR. After extensive counseling, the patient and her husband opted for donor oocyte IVF. The donor oocyte cycle involved synchronizing the patient's endometrium with estrogen and progesterone, retrieving 5 mature oocytes from the donor, and transferring two blastocysts. 14 days post-transfer, a positive βHCG test confirmed successful implantation, leading to a confirmed singleton pregnancy at 7 weeks.
2. Clinical Indication with Hormone Levels :
2.1 Follicle-Stimulating Hormone (FSH):
FSH plays a crucial role in the development and maturation of ovarian follicles. Elevated FSH levels, as seen in the case study where the patient had an FSH level of 23 mIU/mL, often indicate diminished ovarian reserve. According to the ASRM, an FSH level above 10-15 mIU/mL can suggest a reduced ovarian reserve, which can significantly impact the success rates of assisted reproductive technologies (ART) such as IVF [3].
2.2 Anti-Müllerian Hormone (AMH):
AMH is a more reliable marker of ovarian reserve compared to FSH. It is produced by the granulosa cells of the ovarian follicles and reflects the number of primordial follicles. In the case study, the patient's AMH level was 0.252 ng/mL, which is well below the normal range (1-4 ng/mL). Low AMH levels correlate with a decreased number of recruitable follicles and predict a poor response to ovarian stimulation [4].
2.3 β-Human Chorionic Gonadotropin (βHCG):
βHCG is a hormone produced by the placenta after implantation. It is a key marker for early pregnancy detection. In the case study, a positive βHCG test 14 days post-embryo transfer confirmed the successful implantation of the donor oocyte. βHCG levels are typically monitored to assess the viability of the pregnancy and to rule out ectopic pregnancy or other complications [2].
2.4 Additional Biomarkers for Assessing Infertility
a. Estradiol (E2):
E2 is a primary female sex hormone that is essential for the development and maintenance of the female reproductive system. During an IVF cycle, monitoring E2 levels helps in assessing the response to ovarian stimulation. High E2 levels can indicate a good response, while low levels may suggest a poor response [2]. In the case study, the patient's E2 levels were monitored throughout the donor oocyte IVF cycle to ensure optimal endometrial receptivity[1].
b. Progesterone:
Progesterone is critical for preparing the endometrium for implantation and maintaining early pregnancy. In the case of donor oocyte IVF, progesterone supplementation is often required to support the endometrium. The patient in the case study received progesterone to synchronize her endometrium with the donor's cycle, ensuring a conducive environment for embryo implantation [3].
c. Testosterone:
While primarily a male hormone, testosterone also plays a role in female reproductive health. It is involved in the production of E2 and can affect libido and overall reproductive function. However, elevated testosterone levels can lead to polycystic ovary syndrome (PCOS), which is a common cause of infertility [3]. Monitoring testosterone levels can help in diagnosing and managing conditions like PCOS [2].
3. Conclusion
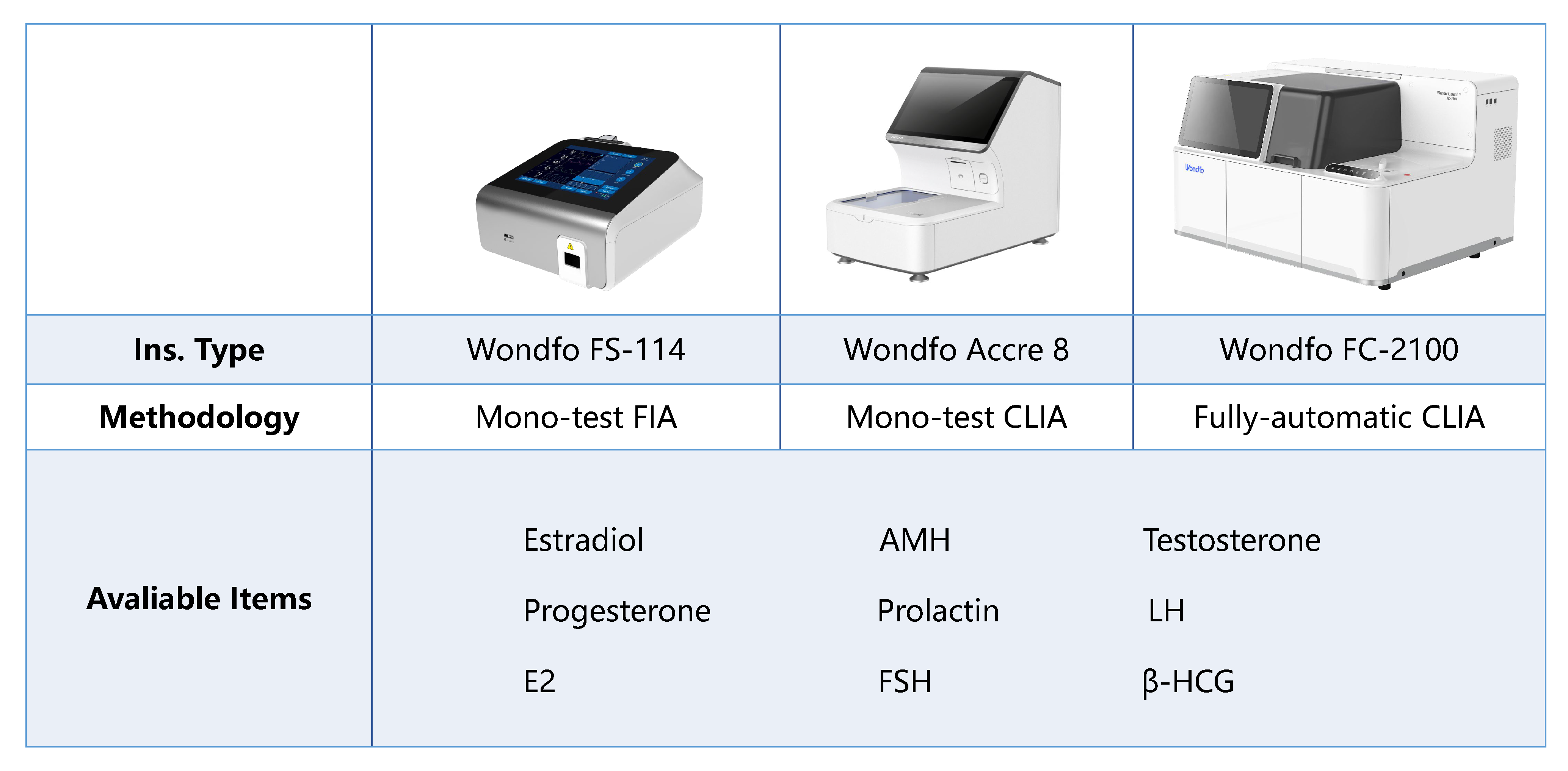
Understanding hormonal markers is crucial for diagnosing and managing female infertility. At Wondfo, we provide innovative and reliable in vitro diagnostic solutions for female infertility. Our comprehensive marker panels on quantitative insights, including FSH, AMH, βHCG, E2, progesterone, and testosterone. Our advanced hormonal assays can similarly identify conditions like diminished ovarian reserve, monitor ovarian stimulation, and ensure optimal endometrial receptivity. Our platforms, from sensitive immunoassays to user-friendly POC devices, enable accurate and timely results, supporting informed clinical decisions and tailored treatment plans.
Reference:
1. Shrivastava, J., More, A., Shrivastava, V., & Shrivastava, D. (2022). Successful outcome in a middle-aged woman with secondary infertility using donor oocyte in vitro fertilization: A case report from a rural infertility clinic. Cureus, 14(8), e27710. https://doi.org/10.7759/cureus.27710
2. Delaney, A., Jensen, J. R., & Morbeck, D. (2021). Fertility testing: How laboratory tests contribute to successful infertility treatments. Clinical Laboratory News, 47(11).
3. Carson, S. A., & Kallen, A. N. (2021). Diagnosis and management of infertility: A review. JAMA, 326(1), 65-76. https://doi.org/10.1001/jama.2021.4788
4. Practice Committee of the American Society for Reproductive Medicine. (2020). Testing and interpreting measures of ovarian reserve: A committee opinion. Fertility and Sterility, 114(6), 1151-1157. https://doi.org/10.1016/j.fertnstert.2020.07.1326
 The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc.
The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc. 50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others.
50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others. Single-dose Chemiluminescense Immunoassay Platform
Single-dose Chemiluminescense Immunoassay Platform Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.
Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously. Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.
Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals. Ready-to-use lyophilized RT-PCR Reagent;
Ready-to-use lyophilized RT-PCR Reagent; Wondfo PA-3600 IHC Staining System
Wondfo PA-3600 IHC Staining System The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds.
The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds. This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.
This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.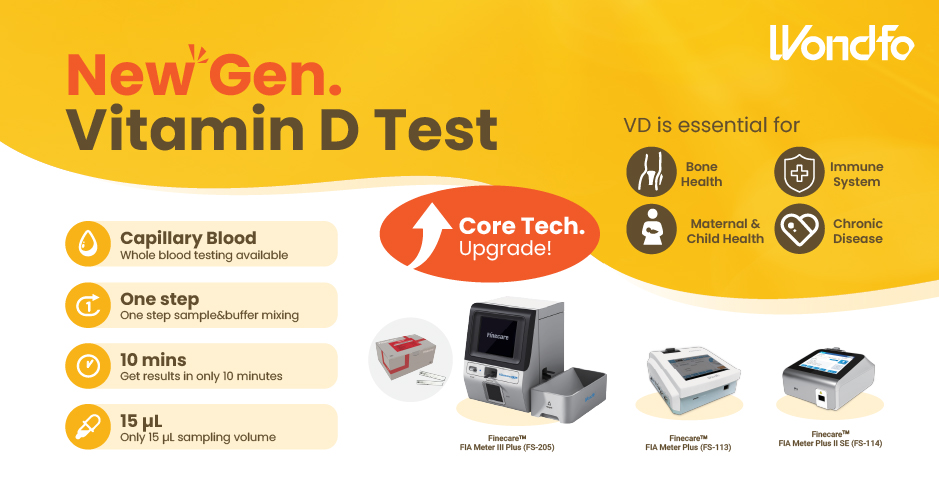 Finecare™ Vitamin D, from complexity to simplicity
Finecare™ Vitamin D, from complexity to simplicity Building A World Free from Antibitoic Overuse
Building A World Free from Antibitoic Overuse Advanced rapid diagnostic test with WHO prequalification for infectious disease
Advanced rapid diagnostic test with WHO prequalification for infectious disease The Future Intelligent Medical Assistant to Healthcare
The Future Intelligent Medical Assistant to Healthcare Fight against the pandemic through continuous innovation
Fight against the pandemic through continuous innovation