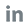1. Introduction to Mpox
Mpox, a zoonotic disease caused by the monkeypox virus (MPXV) from the Orthopoxvirus genus, has seen significant transmission leading to over 70,000 cases worldwide by October 2022 [2]. The evolving epidemiology of mpox raises concerns about its potential to become endemic outside its traditional geographic boundaries. Early and accurate diagnosis is paramount to control the spread and manage the disease effectively [2][4].
2. Diagnostic Methods for Mpox
2.1 NAAT– Gold Standard for Mpox Diagnosis
Currently, nucleic acid amplification testing (NAAT) such as real-time or conventional polymerase chain reaction (PCR) is the gold standard for confirming mpox infection [2]. Skin lesion material, including swabs of lesion exudate, roofs from more than one lesion, or lesion crusts, are used for diagnostic confirmation [2]. When a NAAT result confirms a positive case, WHO recommends sequencing to gain a deeper understanding of viral evolution, transmission chains, and spread patterns. Sequencing plays a crucial role in monitoring viral changes, and the results should be shared in publicly accessible databases to contribute to global knowledge and response efforts.[1]. Despite the robustness of NAAT, there are limitations in accessibility and turnaround time(TAT), particularly in resource-limited settings [1][4].
A potential solution is the POCT PCR method. Wondfo's U-Card Dx instrument integrates nucleic acid extraction, amplification, and analysis into a single device, providing a 'sample in, results out' process. This eliminates the need for a professional PCR lab and specialized personnel, making it ideal for decentralized settings like primary care centers or rural areas. With its compact design, the U-Card Dx delivers results within 28 to 38 minutes, significantly reducing turnaround time and enabling quick patient management to help control disease spread. The device offers high accuracy, demonstrating 100% agreement with traditional PCR methods and a limit of detection (LOD) of ≤1000 copies/mL, making it highly effective for Mpox diagnosis.
2.2 Immunoassays for Mpox
Immunoassay-based methods, such as serology and rapid antigen testing, have limitations in diagnosing mpox, which are hindered by significant cross-reactivity among orthopoxviruses, including Vaccinia, Camelpox, Variola, and Ectromelia viruses. [3] The overlapping antigenicity within the Orthopoxvirus genus complicates differentiation, leading to unreliable diagnostic outcomes and potential false-positive results, especially in individuals who have received vaccinia-based vaccinations. [1] However, considering factors such as testing accessibility, ease of use, and cost, immunoassays, particularly rapid antigen tests, can serve as a viable initial screening tool in scenarios where NAAT is not feasible. Positive results obtained from serology tests need to be confirmed with NAAT.
3. Integration with Current Practices
The World Health Organization's (WHO) technical guidance document titled "Testing for mpox – Individuals and communities" represents the criteria for diagnostic evaluation of mpox. [7] This document emphasizes the identification of clinical signs consistent with mpox infection, including the presence of a characteristic rash or lesions, often preceded or accompanied by systemic symptoms such as fever. It advises healthcare professionals to conduct a thorough risk assessment, incorporating knowledge of local epidemiology and potential exposure risks, to determine the necessity for diagnostic testing. PCR assays are highlighted as the primary method for laboratory confirmation of mpox infections due to their high sensitivity and specificity. Additionally, the document underscores the significance of comprehensive case management, including contact tracing, isolation protocols, and supportive care to mitigate the transmission of the virus within healthcare settings and the community at large. [7]
4. Future Innovations and Collaborations
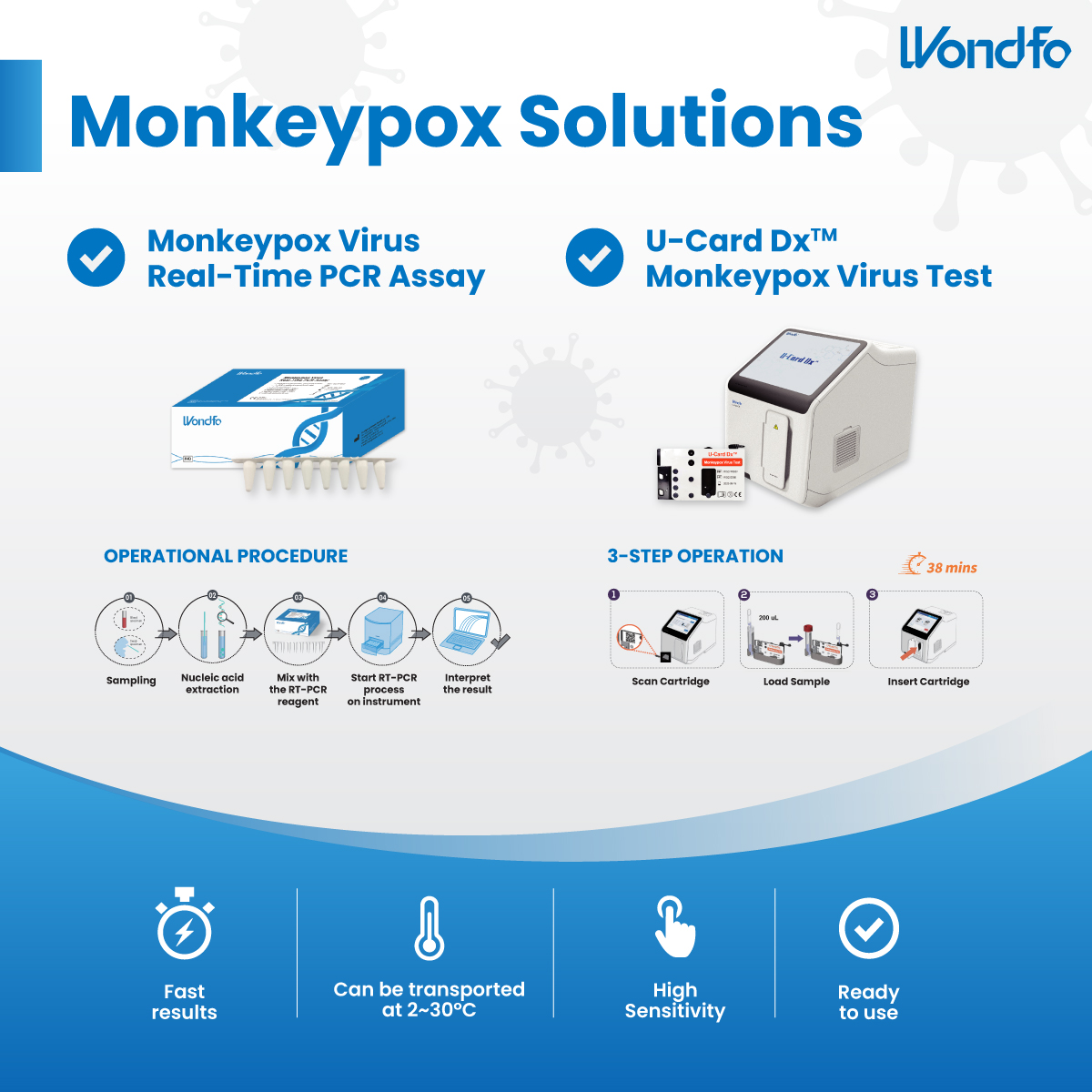
Recently, a new method utilizing the CRISPR/Cas13a detection system and the conception of POCT, SCOPE (Streamlined CRISPR On Poc Evaluation platform), has been developed by Wang [6] and the results have been published on Nature. CRISPR/Cas13a renowned for its capability in gene editing. However, its precision in targeting specific DNA and RNA sequences, presents itself as an exemplary diagnostic modality for infectious diseases. The SCOPE platform incorporates a single-step CRISPR assay that includes recombinase polymerase amplification (RPA), T7 transcription, and CRISPR/Cas13a detection in one tube. The lysed MPXV DNA is amplified by RPA. The double-stranded DNA is labeled with T7 promoters at the 5’ end of the forward primers, enable transcription of single-stranded RNA using T7 RNA polymerase. The RNA products are then recognized by the Cas13a and crRNA complex. Simultaneously, the trans-cleavage activity of Cas13a is activated, leading to the cleavage of the fluorescence-quenching modified single-strand RNA reporter. Results are interpreted by the intensity of fluorescence released from the fluorescence-quenching RNA reporter. The entire procedure includes signal acquisition and result interpretation.
Figure 1: Optimization of essential components of single-step RPA-CRISPR/
Cas13a detection system. [6]
In summary, the significant global spread of mpox underscores the critical need for accurate diagnostic strategies. Conventional PCR offers high sensitivity and specificity, ensuring reliable detection, but its limitations in testing accessibility and delay results. The implementation of POCT in Mpox diagnosis can greatly enhance public health responses by enabling quicker diagnoses and easier access to testing. Significant efforts have been made to develop novel POCT platforms for viral DNA detection, such as the U-Card Dx by the Wondfo team and the SCOPE system. These innovations aim to increase testing accessibility while maintaining diagnostic accuracy, which is crucial in managing epidemics like Mpox.
References:
[1] Lim, C. K., Roberts, J., Moso, M., ... & Williamson, D. A. (2023). Mpox diagnostics: Review of current and emerging technologies. Journal of Medical Virology, 95, e28429. <https://doi.org/10.1002/jmv.28429>
[2] World Health Organization. (2022). Laboratory testing for the monkeypox virus: Interim guidance. World Health Organization. <https://apps.who.int/iris/handle/10665/311529>
[3] Davis, I., Payne, J. M., Olguin, V. L., ... & Ricks, K. M. (2023). Development of a specific MPXV antigen detection immunodiagnostic assay. Frontiers in Microbiology, 14, 1243523. <https://doi.org/10.3389/fmicb.2023.1243523>
[4] European Centre for Disease Prevention and Control. (2024). Risk assessment for the EU/EEA of the mpox epidemic caused by monkeypox virus clade I in affected African countries – 16 August 2024. European Centre for Disease Prevention and Control. <https://ecdc.europa.eu/en/publications-data/risk-assessment-eueea-mpox-epidemic-caused-monkeypox-virus-clade-i-affected>
[5] Osborn, M. J., Bhardwaj, A., Bingea, S. P., Knipping, F., Feser, C. J., Lees, C. J., Collins, D. P., Steer, C. J., Blazar, B. R., & Tolar, J. (2021). CRISPR/Cas9-Based Lateral Flow and Fluorescence Diagnostics. Bioengineering, 8(2), 23. https://doi.org/10.3390/bioengineering8020023
[6] Wang, Y., Chen, H., Lin, K., Han, Y., Gu, Z., Wei, H., Mu, K., Wang, D., Liu, L., Jin, R., Song, R., Rong, Z., & Wang, S. (2024). Ultrasensitive single-step CRISPR detection of monkeypox virus in minutes with a vest-pocket diagnostic device. Nature Communications, 15(3279). https://doi.org/10.1038/s41467-024-47518-8
[7] World Health Organization. (2023). Testing for mpox – Individuals and communities. https://www.who.int/news-room/questions-and-answers/item/testing-for-mpox--individuals-and-communities
 The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc.
The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc. 50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others.
50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others. Single-dose Chemiluminescense Immunoassay Platform
Single-dose Chemiluminescense Immunoassay Platform Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.
Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously. Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.
Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals. Ready-to-use lyophilized RT-PCR Reagent;
Ready-to-use lyophilized RT-PCR Reagent; Wondfo PA-3600 IHC Staining System
Wondfo PA-3600 IHC Staining System The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds.
The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds. This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.
This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers. Finecare™ Vitamin D, from complexity to simplicity
Finecare™ Vitamin D, from complexity to simplicity Building A World Free from Antibitoic Overuse
Building A World Free from Antibitoic Overuse Advanced rapid diagnostic test with WHO prequalification for infectious disease
Advanced rapid diagnostic test with WHO prequalification for infectious disease The Future Intelligent Medical Assistant to Healthcare
The Future Intelligent Medical Assistant to Healthcare Fight against the pandemic through continuous innovation
Fight against the pandemic through continuous innovation