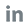1. Introduction
In today's fast-paced healthcare environment, point-of-care testing (POCT) has become a vital tool for improving patient outcomes and streamlining the delivery of medical care. By allowing diagnostic tests to be performed near the patient, POCT facilitates quicker decision-making and intervention. To ensure that POCT results are reliable and accurate, strong quality management systems are crucial. ISO 15189:2022, the latest revision of the international standard for medical laboratories, now includes significant updates that address POCT. This marks a significant milestone as POCT requirements were included in ISO 22870 which will be discontinued upon publication of ISO 15189:2022 [1]. This article explores the integration of POCT requirements in ISO 15189:2022 and interprets them in aspects., emphasizing the importance of staff training and competency assessment for reliable and accurate test results. The revision streamlines accreditation, intensifies proactive risk management, and aligns with broader ISO standards, including ISO/IEC 17025:2017 for testing and calibration laboratories [1].
2. Main Changes from ISO 15189:2012 to ISO 15189:2022
2.1 Personnel Training and Competence
ISO 15189:2022 mandates that laboratories provide necessary training and competency evaluation for all personnel, including those involved in POCT. Chapter 6.2.1 and Annex.4 in the updated version emphasize the importance of targeted training to ensure that POCT personnel are competent in POCT procedures and operations [1]. Digital solutions can facilitate the efficient tracking and management of personnel training records, ensuring that all staff members are up-to-date with the latest POCT protocols and requirements [2]. Professional certification programs, such as the one described by Isbell et al. [4], provide a standardized approach to verifying the competence of POCT personnel.
2.2 Facilities and Environmental Conditions
As per chapter 6.3.1 in ISO 15189:2022, facilities and environmental conditions must be suitable for laboratory activities, including POCT, and not adversely affect the validity of results or the safety of patients, visitors, laboratory users, and personnel [1]. This includes pre-examination related facilities and POCT sites, ensuring that they are designed and maintained to support accurate and safe testing.
2.3 Reagents and Consumables Management
ISO 15189:2022 requires processes for the selection, procurement, reception, storage, acceptance testing, and inventory management of reagents and consumables, including POCT supplies [1]. This ensures that the quality and integrity of reagents and consumables used in POCT are maintained throughout their life cycle, supporting the reliability of test results.
2.4 Service Agreements and Collaboration
Service agreements between the laboratory and other parts of the organization using laboratory-supported POCT are essential. These agreements ensure that respective responsibilities and authorities are clearly specified and communicated, facilitating effective collaboration and coordination [1]. ISO 15189:2022 suggests the use of established multidisciplinary POCT committees to manage such service agreements, promoting a structured approach to managing POCT activities. Digital tools can assist in the management of service agreements by providing a centralized platform for communication and documentation, enhancing collaboration between different stakeholders.
2.5 Examination Processes and Quality Assurance
Building on the existing requirements for quality management systems outlined in ISO 15189, the updated version (ISO 15189:2022) emphasizes the need for following established procedures and recording the identity of personnel, including POCT operators. It also highlights the importance of participating in external quality assessment (EQA) programs for POCT examination methods and appointing a responsible person with appropriate training and experience for POCT quality [1]. Digital platforms can support these requirements by automating the recording of examination processes and ensuring compliance with established procedures. Additionally, they facilitate participation in EQA programs by providing tools for data collection and analysis, ensuring that POCT end-users can easily track and demonstrate their compliance with ISO 15189:2022 [3]. Ricós et al. [5] discuss the evolution of internal quality control (IQC) and the importance of implementing robust IQC protocols to ensure the reliability of POCT results.
3. Conclusion
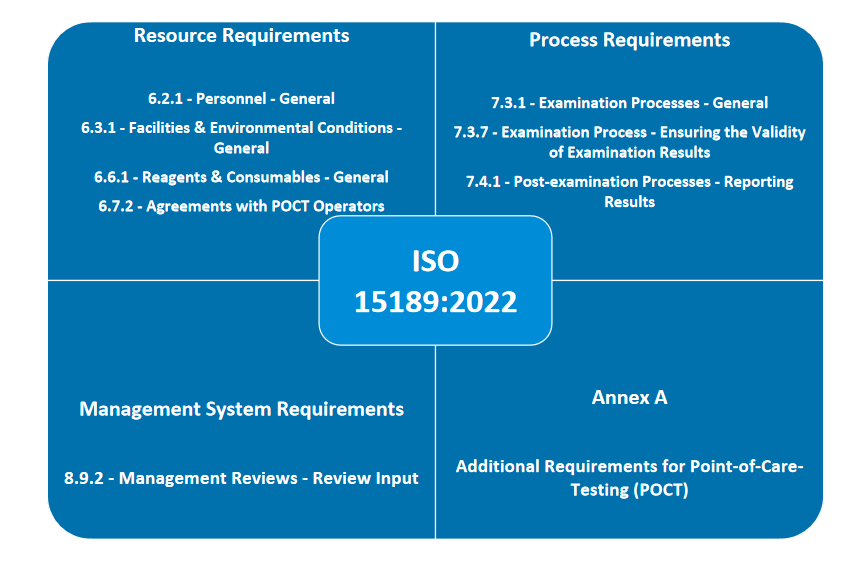
The update of ISO 15189 is essential for maintaining the quality and competence of POCT. As POCT continues to grow, adherence to these standards is vital for delivering high-quality patient care. Laboratories must effectively integrate these standards to manage their POCT programs effectively, ensuring they meet robust quality criteria and foster ongoing improvement. For laboratories looking to implement these standards effectively, Wondfo offers a comprehensive POCT solution that includes not only a broad range of POCT products such as instruments, internal quality control reagents but also a cloud-based information system. This integrated solution is designed to support the seamless integration of POCT into the overall quality management system. The cloud-based information system provides tools for personnel management, reagent and consumable tracking, examination process automation, and management reviews. By leveraging this comprehensive solution, laboratories can streamline their operations, enhance compliance, and improve patient outcomes.
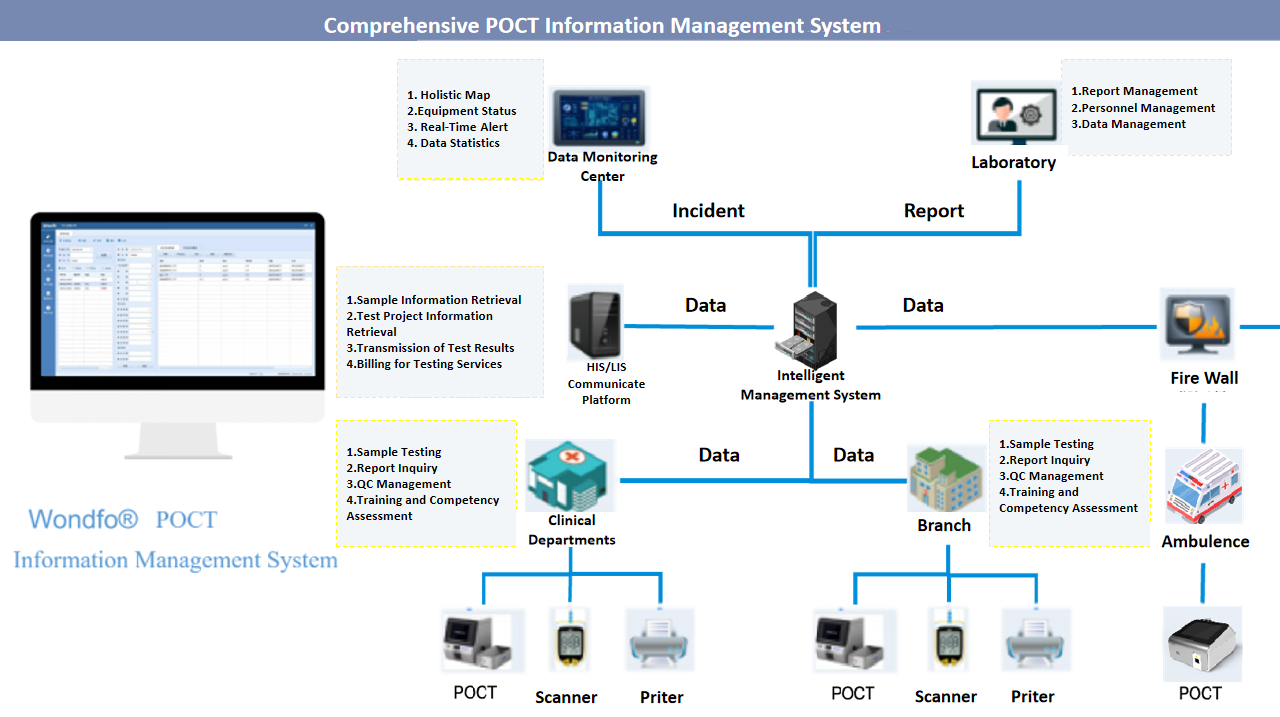
Wondfo 1m² POCT lab (integration of POCT products and information management system)
Reference:
1. International Organization for Standardization. (2022). ISO 15189:2022 Medical laboratories — Requirements for quality and competence. https://www.iso.org/standard/83238.html
2. Fung, A. W. S. (2020). Utilizing connectivity and data management system for effective quality management and regulatory compliance in point of care testing. Practical Laboratory Medicine, 22, e00187. https://doi.org/10.1016/j.plabm.2020.e00187
3. Yenice, S. (2021). Training and competency strategies for point-of-care testing. eJIFCC, 32(2), 167-178. https://doi.org/10.1515/almed-2021-0029
4. Isbell, T. S., Colwell, E., Frank, E. L., Karon, B. S., Luzzi, V., & Wyer, L. A. (2021). Professional certification in point-of-care testing. eJIFCC, 32(3), 303-310. https://doi.org/10.1515/almed-2021-0029
5. Ricós, C., Fernández-Calle, P., Perich, C., & Westgard, J. O. (2022). Internal quality control – past, present and future trends. eJIFCC, 32(3), 303-310. https://doi.org/10.1515/almed-2022-0029
6. Brun, M., Füzér, A. K., Henschke, B., Rozak, K., & Venner, A. A. (2021). Identifying sources of error and selecting quality indicators for point of care testing. eJIFCC, 32(2), 131-139. https://doi.org/10.1515/almed-2021-0029
7. de la Villa Porras, I. (2023). Novelties in the ISO 15189:2023 standard. eJIFCC, 32(3), 303-310. https://doi.org/10.1515/almed-2023-0139
 The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc.
The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc. 50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others.
50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others. Single-dose Chemiluminescense Immunoassay Platform
Single-dose Chemiluminescense Immunoassay Platform Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.
Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously. Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.
Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals. Ready-to-use lyophilized RT-PCR Reagent;
Ready-to-use lyophilized RT-PCR Reagent; Wondfo PA-3600 IHC Staining System
Wondfo PA-3600 IHC Staining System The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds.
The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds. This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.
This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.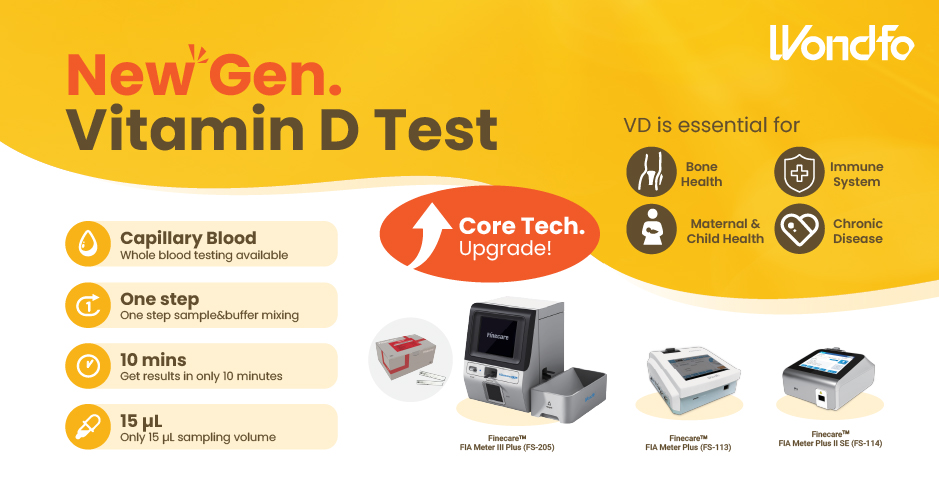 Finecare™ Vitamin D, from complexity to simplicity
Finecare™ Vitamin D, from complexity to simplicity Building A World Free from Antibitoic Overuse
Building A World Free from Antibitoic Overuse Advanced rapid diagnostic test with WHO prequalification for infectious disease
Advanced rapid diagnostic test with WHO prequalification for infectious disease The Future Intelligent Medical Assistant to Healthcare
The Future Intelligent Medical Assistant to Healthcare Fight against the pandemic through continuous innovation
Fight against the pandemic through continuous innovation