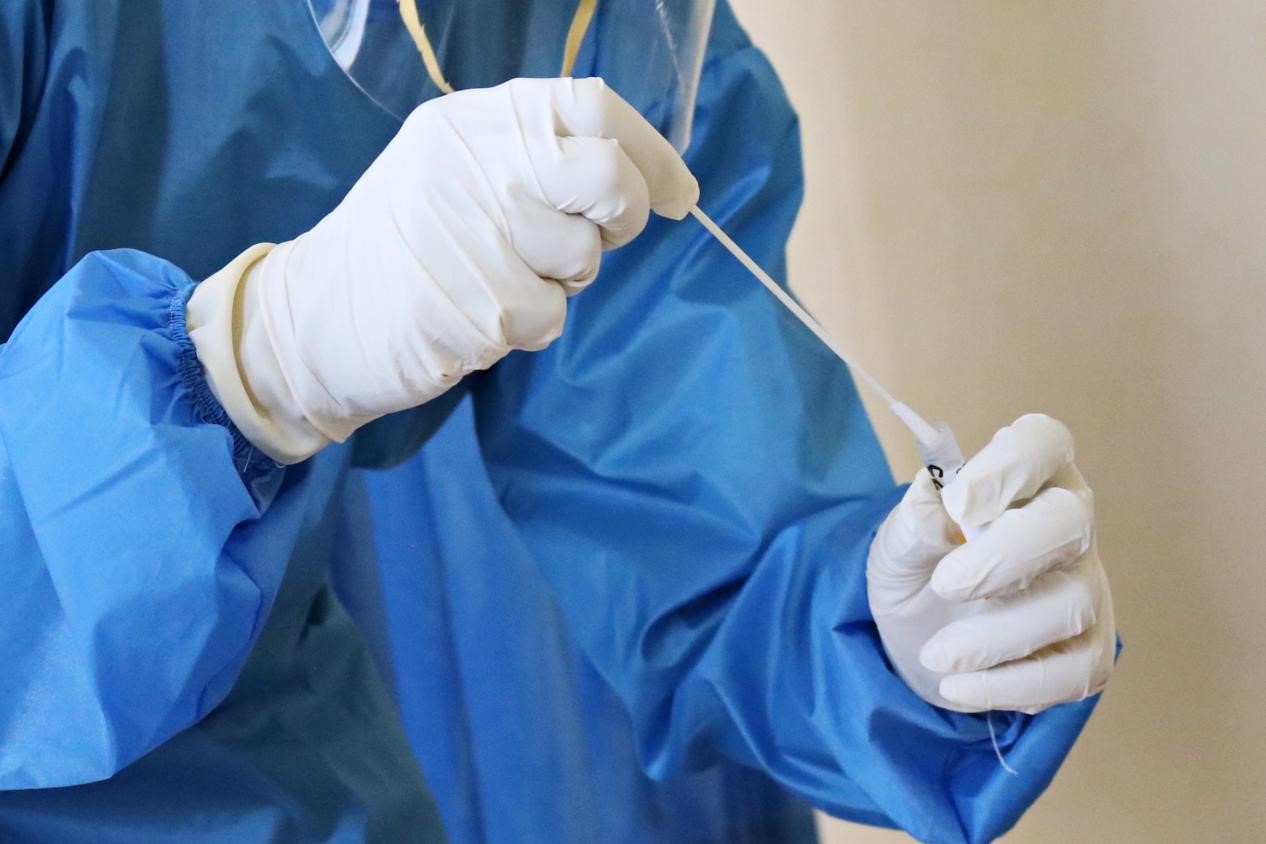
Testing is a critical element to the overall prevention and control strategy for COVID-19. As of July 2021, the United States has carried out the third most COVID-19 tests per 1 million population among the countries most severely impacted by the pandemic. The U.S. has performed roughly 526 million SARS-CoV-2 tests in total, according to the latest Statista reports.
Screening and diagnostic testing for SARS-CoV-2 are critical components to the overall prevention and control strategy for COVID-19. Understanding the differences between tests in terms of their intended use and performance is paramount to achieve positive health outcomes for the individual and the population as a whole. Here is a quick guide on how to distinguish screening from diagnostic testing.
COVID-19 Screening tests
According to the FDA fact sheet “Screening for COVID-19”, COVID-19 screening tests are used to identify individual infections in a group, even if there is no suspicion of a COVID-19 infection.
Based on the recent testing guidelines from the Centers for Disease Control and Prevention, screening tests are recommended for unvaccinated people to identify those who are asymptomatic and do not have known or suspected exposure to COVID-19. Screening involves the identification of unknown cases so that measures can be implemented to prevent further transmission.
Since every COVID test features a different turnaround time, their performance characteristics may vary. Based on preliminary
BMJ reports, the accuracy of the tests is found by comparing their results with a gold standard, often using viral RNA detection with PCR testing when the symptoms occur. However, the sensitivity in PCR testing could be as low as 70%. The maximum sensitivity for combined Immunoglobulin tests (IgG & IgM) was 96% at 22 to 35 days after the onset of COVID symptoms.
However, according to Harvard Health Publishing, antibody tests have a 20% rate of false negatives. Test results may differ based on the time when the test is carried out. It takes several days for the human body to generate antibodies against the virus. Furthermore, the level of antibodies fluctuates in the next few months post-infection. Therefore, the range of false negatives can be anywhere from 0-30%. Although testing positive signifies a person was exposed to the pathogen, it may not indicate long-term protective immunity or lack of contagiousness.
COVID-19 screening tests are not used to diagnose the illness. They are meant to detect it early on and encourage those affected to change their lifestyle, explains the
American Society for Microbiology.
COVID-19 Diagnostic tests
COVID-19 diagnostic tests spot the current infection. They are performed on patients with symptoms or signs of an infection. COVID-19 diagnostic tests or viral tests can also be proposed for asymptomatic patients that were exposed to the virus.
These include:
-
NAATs (nucleic acid amplification tests) – featuring 80% sensitivity and 99% specificity, these tests are very accurate, research shows. If coronavirus prevalence is 20%, the false negatives rates increase with prevalence. These tests take 1 to 3 days for results. The turnaround time can take less than an hour to over a day.
-
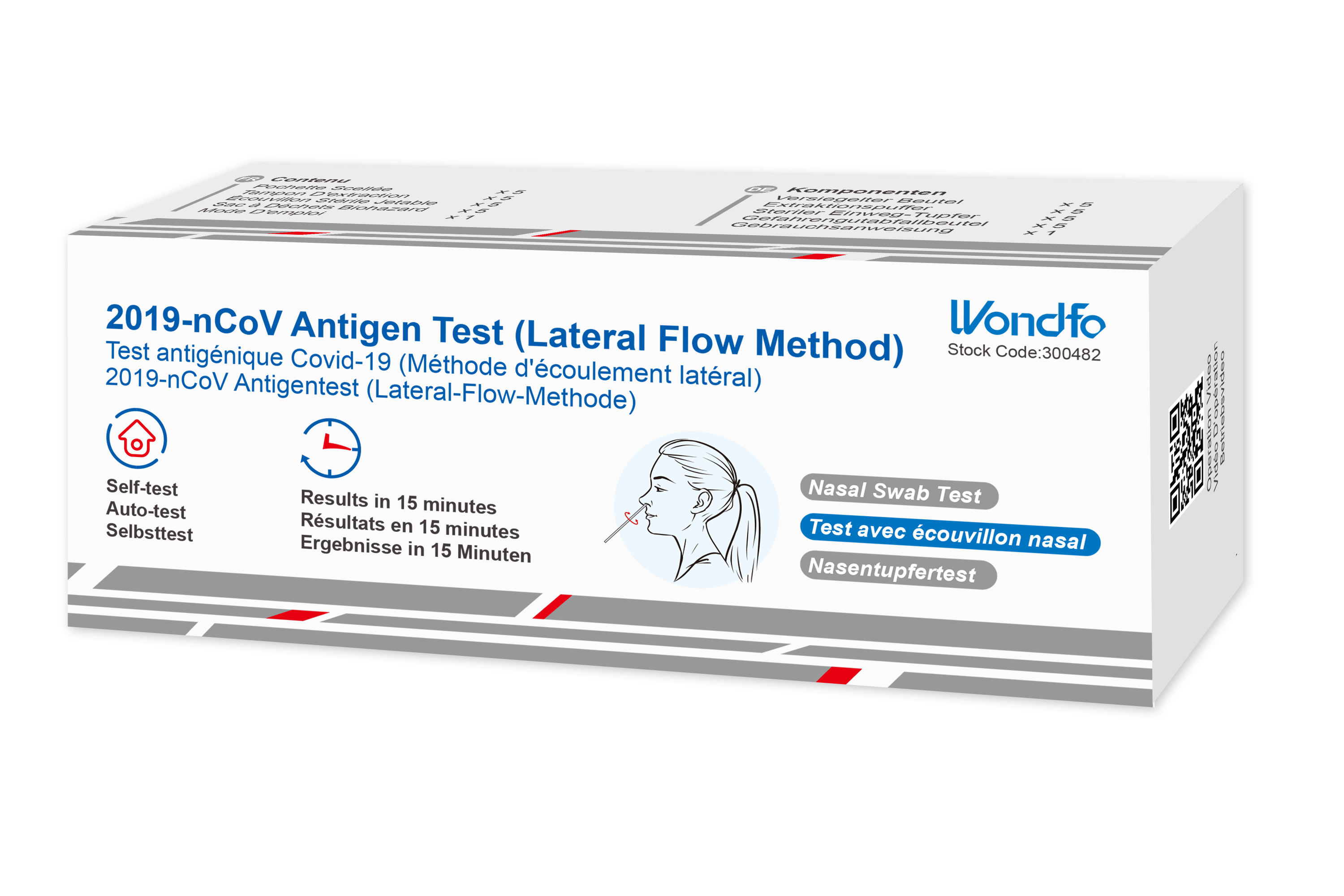
Antigen tests – with a specificity quite similar to NAATs, antigen tests are less sensitive and more affordable than NAATs. Their turnaround time is 15-30 min. However, their results are presumptive, stated the CDC. Roughly 70 out of 100 positive tests can be false positives, explains the FDA.
The US Centers for Disease Control and Prevention does not recommend the use of antibody tests to diagnose active infections. Antibody tests can detect antibodies to the COVID-19 virus following an individual’s infection or vaccination.
Comparison of Diagnostic & Screening Tests
COVID-19 diagnostic tests are employed to establish an absence (or a presence) of the pathogen. COVID-19 screening tests, on the other hand, are meant to detect the possible risk factors or predisposition for that pathogen. They can also be used to monitor the early stages of the disease, experts explain.
COVID-19 screening tests target a broader population. People are broadly selected and get tested, whereas diagnostic testing focuses on those who had a positive screening test. Diagnostic tests use invasive maneuvers but for a justifiable cause. Their results are definite and come at a higher price.
The screening is simpler and tailored towards the general public. However, it warrants further confirmation. So, even if a patient tests positive, they will need to get a definite diagnosis. The idea of a screening test is to spot potential cases. This can aid patients in avoiding or managing the illness.
For large-scale screening, antigen testing is the most suitable solution. Antigen tests can be utilized in sensitive areas for rapid screening, such as airports, schools, and workplaces. Thanks to their short turnaround time (approximately 15 minutes), rapid antigen tests can detect infected individuals, preventing the further spreading of the virus.
Conclusion
COVID testing is now more critical than ever. With the unpredictable development of COVID-19 epidemic “waves”, it is crucial to dispose of all the testing methods that best suit the individual’s specific scenario to stop and prevent the spread of the virus.
Each type of test has something to offer, but all the various testing strategies share a common goal of curbing the spread of COVID-19. The information listed here above reflects the state of research on the SARS-CoV-2 epidemic at the time of writing. In addition to rigorous testing and prevention measures, the advancement of scientific knowledge and research is equally as important to control the spread of COVID-19.
For Wondfo Biotech, research is not a buzzword. As China's POCT industry leader with rapid tests sold to over 140 countries and regions, Wondfo’s mission is to safeguard the health and improve the lives of people worldwide. Since its foundation in 1992, Wondfo Biotech has invested in the R&D, production, and sales of professional rapid diagnosis and chronic disease management solutions.
Over 706 to-notch researchers at Wondfo work incessantly to innovate and develop solutions that meet the present and emerging needs in the POCT and healthcare industries. Wondfo lives by its innovation, as exemplified by its “Nine Technology Platforms”. This product system includes solutions developed to address the latest challenges of nine core diagnostic techniques: the immune colloidal gold, immune-fluorescence, electrochemistry, dry biochemistry, chemiluminescence, molecular diagnostics, instrument, pathology, and biological raw material.
Click
here to discover Wondfo’s complete COVID-19 diagnostic solutions.
Racing for Life
About Wondfo
Wondfo, founded in 1992, is the leader of China's point-of-care testing (POCT) industry. We now own multiple advanced technology platforms including immune colloidal gold, immunofluorescence, electrochemistry, dry biochemistry, chemiluminescence, molecular diagnostics, pathology, and have a wide range of products for the rapid identification of cardiovascular disease, inflammation, tumor, infectious disease, drug of abuse, pregnancy, etc. Our products are widely recognized in over 140+ countries.
Our commitment is to make the next breakthroughs in healthcare, providing communities worldwide with life-changing technology to ensure everybody's well-being.
 The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc.
The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc. 50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others.
50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others. Single-dose Chemiluminescense Immunoassay Platform
Single-dose Chemiluminescense Immunoassay Platform Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.
Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously. Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.
Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals. Ready-to-use lyophilized RT-PCR Reagent;
Ready-to-use lyophilized RT-PCR Reagent; Wondfo PA-3600 IHC Staining System
Wondfo PA-3600 IHC Staining System The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds.
The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds. This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.
This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers. Finecare™ Vitamin D, from complexity to simplicity
Finecare™ Vitamin D, from complexity to simplicity Building A World Free from Antibitoic Overuse
Building A World Free from Antibitoic Overuse Advanced rapid diagnostic test with WHO prequalification for infectious disease
Advanced rapid diagnostic test with WHO prequalification for infectious disease The Future Intelligent Medical Assistant to Healthcare
The Future Intelligent Medical Assistant to Healthcare Fight against the pandemic through continuous innovation
Fight against the pandemic through continuous innovation