Recently, Wondfo Biotech was awarded with EU quality management system certificate (IVDR) by TÜV SÜD. Besides, Wondfo’s products has obtained IVDR CE certificates such as HbA1c test kits (immunofluorescence assay) and C-reactive protein test kits (immunofluorescence assay).
Among these certificates, Wondfo’s IVDR quality management certificate is the first that is awarded to a Chinese company, and HbA1c test kits and CRP test kits are in the first batch of Chinese NPT products (Near Patient Testing) that have gained IVDR CE qualification.
With the IVDR quality management system certificated, Wondfo becomes a medical device manufacturer approved by IVDR CE—a newly launched EU regulation on medical devices. This achievement is a solid cornerstone for Wondfo to adapt to new procedures of CE qualification.
The certificate awarded is the first batch that Wondfo has gained under the IVDR CE system. Products mentioned above are the first batch of Chinese NPT products that have gained IVDR CE qualification, under C-class supervision. According to EU regulation, supervision on medical devices can be divided into four classes—A, B, C and D—among which D is the strictest class. Under stricter supervision, products above can be sold for use in both professional labs and POCT scenarios, which can exert positive influence on Wondfo’s businesses in EU member countries.
As a “visa” of products into European market, CE qualification is an obligatory requirement. IVDR EU 2017/746 will be wholly applied to EU countries from May 26
th, 2022.
After the official implementation of IVDR, stricter requirements will be applied in technical documents inspection, clinical assessment, supervision of public companies. It represents stronger regulation of EU on medical devices, as well as a more consistent standard of medical devices regulation in various EU members.
Racing for Life
About Wondfo
Wondfo, founded in 1992, is the leader of China's point-of-care testing (POCT) industry. We now own multiple advanced technology platforms including immune colloidal gold, immunofluorescence, electrochemistry, dry biochemistry, chemiluminescence, molecular diagnostics, pathology, and have a wide range of products for the rapid identification of cardiovascular disease, inflammation, tumor, infectious disease, drug of abuse, pregnancy, etc. Our products are widely recognized in over 140+ countries.
Our commitment is to make the next breakthroughs in healthcare, providing communities worldwide with life-changing technology to ensure everybody's well-being.
 The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc.
The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc. 50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others.
50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others. Single-dose Chemiluminescense Immunoassay Platform
Single-dose Chemiluminescense Immunoassay Platform Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.
Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously. Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.
Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals. Ready-to-use lyophilized RT-PCR Reagent;
Ready-to-use lyophilized RT-PCR Reagent; Wondfo PA-3600 IHC Staining System
Wondfo PA-3600 IHC Staining System The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds.
The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds. This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.
This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.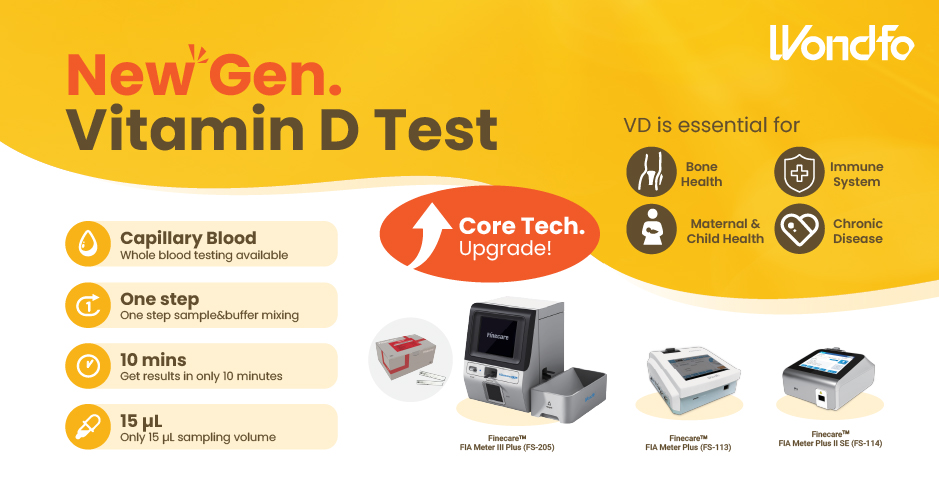 Finecare™ Vitamin D, from complexity to simplicity
Finecare™ Vitamin D, from complexity to simplicity Building A World Free from Antibitoic Overuse
Building A World Free from Antibitoic Overuse Advanced rapid diagnostic test with WHO prequalification for infectious disease
Advanced rapid diagnostic test with WHO prequalification for infectious disease The Future Intelligent Medical Assistant to Healthcare
The Future Intelligent Medical Assistant to Healthcare Fight against the pandemic through continuous innovation
Fight against the pandemic through continuous innovation