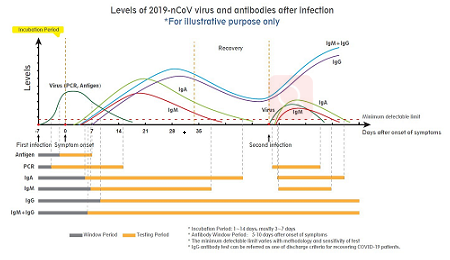
Adhering to the philosophy of “Racing for Life”, Wondfo has been continuously working to satisfy the global demand for rapid tests in the prevention and control of pandemics worldwide. Wondfo 2019-nCoV Antigen Test (Lateral Flow Method) which is performed with a swab sample from the nasal cavity delivers results in 15 minutes, significantly shortening the detection time, comparing to the PCR method. The test can be used by laypeople with the help of the instructions for use and gives users high flexibility with very good quality results.
German Health Minister Jens Spahn said the approval of COVID-19 antigen tests for self-administration allows larger population to get tested. The early identification of asymptomatic individuals can efficiently break the chain of infection, stopping the spread of infection. Under this strategy, a positive at-home test result should be then subjected to a laboratory nucleic acid test. At present in Germany, individuals are required to get tested at home twice a week, increased from once a week earlier. In the key populations, as often as five times a week is required.
As the only company that holds both certificates of the 2019-nCoV antigen test and the 2019-nCoV antibody test, Wondfo is committed to making contributions to the global battle against the pandemics. Wondfo’s multiple COVID-19 tests have achieved CE mark approval and been validated by the importer's country according to the local standards and regulations. Wondfo is now providing a “PCR+ Antigen+ Antibody” integrated solution that meets various application scenarios of on-spot diagnosis of COVID-19 infection.
Wondfo, a POCT leader in China, now owns multiple advanced technology platforms including immune colloidal gold, immunofluorescence, electrochemistry, dry biochemistry, chemiluminescence, molecular diagnostics, pathology, etc.
Based on the above technology platforms, Wondfo has extended the product lines to the rapid identification of cardiovascular diseases, inflammation, tumor, infectious diseases, drug abuse, pregnancy, etc.
The products are widely used in clinical laboratory diagnosis, primary care, the effective management of critical and severe diseases, pandemics prevention and control, forensic expertise, personal healthcare, and other fields, widely sold to 140+ countries and regions.
 The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc.
The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc. 50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others.
50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others. Single-dose Chemiluminescense Immunoassay Platform
Single-dose Chemiluminescense Immunoassay Platform Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.
Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously. Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.
Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals. Ready-to-use lyophilized RT-PCR Reagent;
Ready-to-use lyophilized RT-PCR Reagent; Wondfo PA-3600 IHC Staining System
Wondfo PA-3600 IHC Staining System The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds.
The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds. This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.
This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.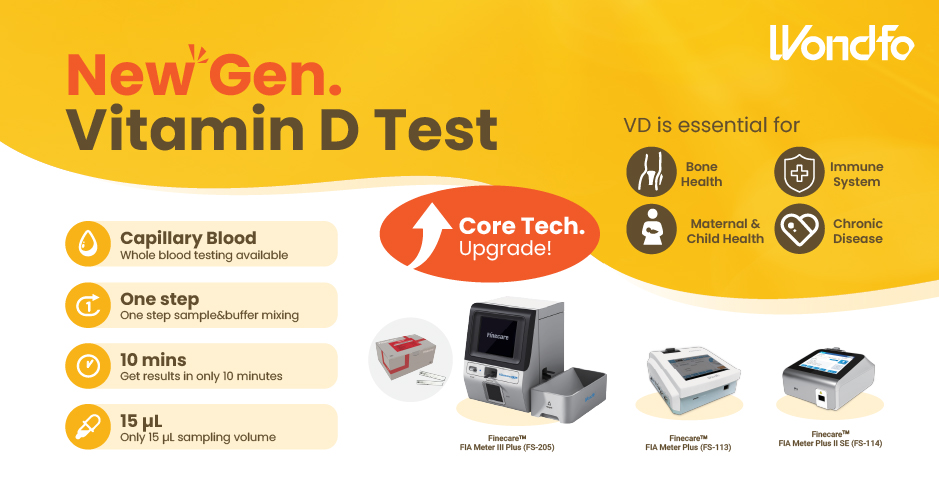 Finecare™ Vitamin D, from complexity to simplicity
Finecare™ Vitamin D, from complexity to simplicity Building A World Free from Antibitoic Overuse
Building A World Free from Antibitoic Overuse Advanced rapid diagnostic test with WHO prequalification for infectious disease
Advanced rapid diagnostic test with WHO prequalification for infectious disease The Future Intelligent Medical Assistant to Healthcare
The Future Intelligent Medical Assistant to Healthcare Fight against the pandemic through continuous innovation
Fight against the pandemic through continuous innovation