Wondfo is ramping up neutralizing antibody, antigen and PCR tests to address the evolving demands for COVID-19 testing globally. As countries draw up plans to deal with the new wave of infections amidst the pandemic while vaccination programmes are rolled out, Wondfo remains resolute and agile to provide healthcare authorities with the specific tests they need to apply appropriate testing strategies that can help bring the pandemic under control.
Testing is crucial as it informs effective mass intervention programmes that can also minimise disruption to economic activities. However, during a pandemic of such size and scale, it is difficult for countries to determine a perfect testing strategy, as there is no one-size-fits-all approach. It is not only impossible for countries to rely on one 'gold standard' test, they cannot adopt the exact same strategies due to varying demographics, infrastructure, cultural attitudes, and economic capacity.
Testing is a nuanced process. When determining which type of test to use, key aspects such as changes in the course of the disease and the nature of the tests available need to be incorporated.
Neutralizing antibody testing, antigen testing and PCR key in battle against COVID-19 in 2021
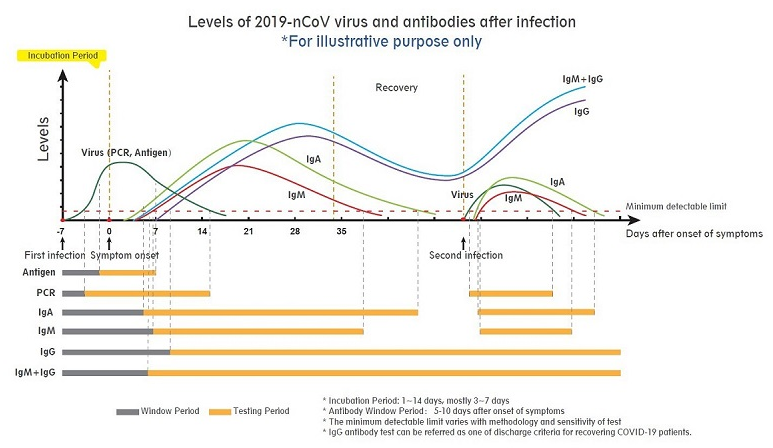
Depending on the time of the appearance of coronavirus and the presence of the two types of antibodies in the human body (IgG or IgM) designed to fight it, PCR and antigen tests are suitable tests in the early onset period (WHO guidelines recommend the use of PCR and antigen tests for triage), while antibody tests are suitable later on when antibodies are produced over days to weeks after infection with the virus and in even larger numbers with a second infection. In addition, the studies of neutralizing antibodies, those that prevent or treat disease, can aid in the development and evaluation of vaccines.
PCR tests are considered the 'gold standard' for COVID-19 testing. The WHO has advised PCR tests should be the recommended method for the identification and laboratory confirmation of COVID-19 cases. However, PCR requires laboratory processing techniques and can take up to 48 hours. In comparison, antigen and antibody tests, also known as rapid tests, can return a result in 15-30 minutes and play a valuable role in screening, disease surveillance and epidemiologic research.
Where countries are operating testing programmes under less-than-ideal circumstances due to the rapid spread of the virus and a lack of medical resources, rapid tests are also used alongside PCR tests in settings where they may be the best available option.
Wondfo's unremitting effort to offer support in the battle against the pandemic
Operating in an emerging and evolving battlefield, Wondfo holds itself to the highest standards. Wondfo has been working around the clock in collaboration with research institutions and healthcare authorities to provide testing solutions and products that meet their specific and urgent needs.
Last year, studies by researchers at Brown University and the University of California showed that Wondfo's antibody tests achieved the highest sensitivity and specificity among all the tests evaluated. Researchers and hospital labs in Brazil, France and Australia have also shared the same conclusions and recommended the tests in national and regional testing programmes. As of today, Wondfo has supplied more than 50 million COVID-19 tests in 93 countries since the beginning of the pandemic.
Wondfo's antibody tests have been selected as the main test assays by healthcare authorities across a diverse range of testing scenarios and played a critical role in the national pandemic response in Spain, Brazil, Ireland and Indonesia. Wondfo's tests were used by the Brazilian Ministry of Health in population-based surveys in 133 sentinel cities across all Brazilian states. The data will inform decisions on preventive policies and health system preparedness at the state level.
The same tests were used in a Brazilian study of COVID-19 in children to detect if a child has a post-infectious syndrome caused by an infection i.e. Multisystem Inflammatory Syndrome in Children or MIS-C.
Wondfo is also providing antigen tests to healthcare regimes in 38 countries. Antigen test, a type of rapid test that detects the presence of viral proteins (antigens), is best used to quickly detect acute infection when the viral load is high or when a person has a known exposure to infection, and can be used for early triage and inform control measures of suspected populations.
As vaccination programmes get rolled out, Wondfo has also observed the rising demand for neutralizing antibody tests. The ability to detect and study neutralizing antibodies helps people understand and determine their effect on the human body and thus are essential for vaccine efficacy evaluation and measuring immune response in communities.
As the world continues to battle the pandemic in 2021, fighting original and mutated viruses, Wondfo believes neutralizing antibody testing, antigen testing and PCR testing will be critical to support the containment of disease and the development of vaccines and thus hold the keys to bring the pandemic under control. With comprehensive testing solutions – Wondfo's RT-PCR and antigen tests can detect the new B.1.1.7 and B.1.351 coronavirus strains and wild-type 2019-nCoV – Wondfo remains committed to providing high quality products that can contribute to the global effort.
About Wondfo
Guangzhou Wondfo Biotech Co., Ltd., has been focusing on the R&D, production, sales and service of point-of-care testing (POCT) products and providing customers with professional rapid diagnosis and chronic disease management solutions since founded in 1992.
Wondfo now has more than 4000 employees and a wide range of products for the rapid identification of cardiovascular diseases, inflammation, tumor, infectious diseases, drug abuse, pregnancy, and so on, widely sold to 140+ countries and regions. For more information, please visit
http://en.wondfo.com.cn/ or contact sales@wondfo.com.cn.
 The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc.
The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc. 50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others.
50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others. Single-dose Chemiluminescense Immunoassay Platform
Single-dose Chemiluminescense Immunoassay Platform Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.
Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously. Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.
Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals. Ready-to-use lyophilized RT-PCR Reagent;
Ready-to-use lyophilized RT-PCR Reagent; Wondfo PA-3600 IHC Staining System
Wondfo PA-3600 IHC Staining System The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds.
The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds. This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.
This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.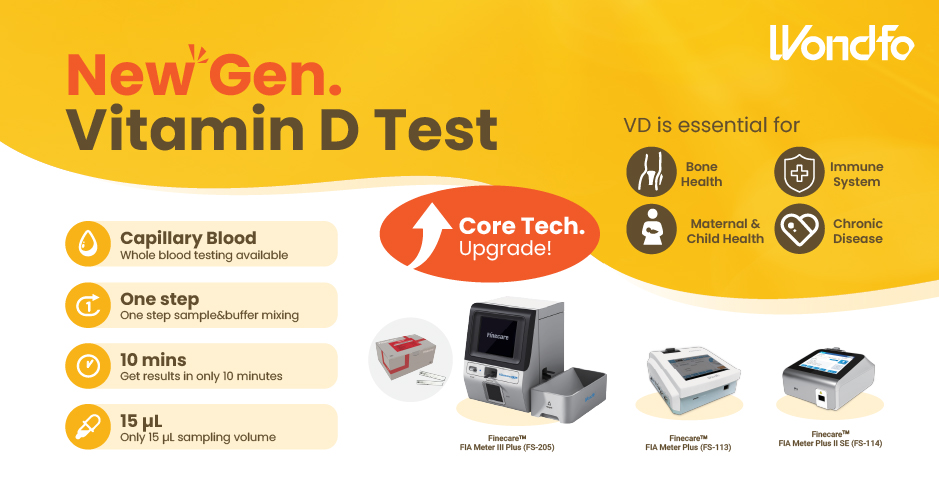 Finecare™ Vitamin D, from complexity to simplicity
Finecare™ Vitamin D, from complexity to simplicity Building A World Free from Antibitoic Overuse
Building A World Free from Antibitoic Overuse Advanced rapid diagnostic test with WHO prequalification for infectious disease
Advanced rapid diagnostic test with WHO prequalification for infectious disease The Future Intelligent Medical Assistant to Healthcare
The Future Intelligent Medical Assistant to Healthcare Fight against the pandemic through continuous innovation
Fight against the pandemic through continuous innovation