On October 24, Wondfo Biotech (300482.SZ) released its third-quarter financial report for 2024. During the first three quarters, the company achieved a revenue of RMB 2.181 billion, an increase of 8.83% year-on-year (YoY). Net profit attributable to shareholders reached RMB 436 million, up 9.04% YoY, with net cash flow from operating activities surging by 577.95% to RMB 173 million. In Q3 alone, Wondfo reported revenue of RMB 606 million, a 17.51% increase YoY, and net profit of RMB 80.14 million, up 22.70%, reaching historical highs in both revenue and profit.
Amid fierce market competition, Wondfo has increased R&D investment to drive innovation in POCT and IVD. R&D expenses reached RMB 271 million in the first three quarters, accounting for 12.43% of revenue. The company accelerates global market penetration, expands overseas installations, and expedites product registration and localization efforts in key international markets.
Since 2002, Wondfo has been actively developing international markets, with thousands of products sold in over 150 countries and regions. Throughout 2024, the company showcased new products at international medical exhibitions, including platforms like chemiluminescence, immunofluorescence, electrochemistry, and molecular diagnostics. New launches included the fully-auto chemiluminescence analyzer FC-2100, the fluorescence Finecare™ FIA Meter X Series, and the automatic blood gas analyzer Ucare-6000.
In North America, Wondfo has made strides in securing registrations for core products in drug testing and infectious disease diagnostics. In H1 2024, the WELLlife™ COVID-19 / Influenza A&B Test, and its Home Test both received EUA (Emergency Use Authorization) from the U.S. FDA, laying a solid foundation for deeper market penetration. In H2, the SAFElife™ Fentanyl Urine Test and Home Test received FDA 510(k) approval, supporting efforts to combat the global opioid crisis. Additionally, the WELLlife™ COVID-19 Antigen Home Test received 510(k) approval, expanding its portfolio and business opportunities.
Wondfo is also pursuing new partnerships to enhance global public health. In July 2024, Wondfo's Malaria P.f (HRP2/pLDH) Test obtained WHO Prequalification (PQ) certification, joining the list of recommended in-vitro diagnostic products. This milestone follows similar PQ approvals for Wondfo's HIV professional-use and self-test products, further validating the company's product quality and management systems within the WHO procurement framework. Wondfo is exploring collaboration with organizations like MedAccess to advance malaria diagnostics and improve global malaria control efforts.
In terms of localized production, Wondfo has achieved significant progress. After establishing a plant in Uganda last year, the company signed a cooperation agreement in August 2024 with Nigerian Presidential Initiative on Healthcare Value Chain (PVAC). This agreement will enhance Wondfo's supply chain resilience across Africa. Under the terms, the Nigerian government will provide policy, financial, and infrastructure support to facilitate the local production of Wondfo's diagnostic reagents.
Looking ahead, Wondfo will continue to pursue its strategy of "Dual-Engine Synergy, Product Leadership, Quality Growth, and Strengthened Organizational Capacity". With a focus on stable growth, the company will accelerate its core businesses across domestic, international, and North American markets. By bolstering its competitiveness in immunodiagnostics and developing molecular diagnostics as a new growth engine, Wondfo aims to become a leading international enterprise with innovative technology and superior products.
 The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc.
The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc. 50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others.
50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others. Single-dose Chemiluminescense Immunoassay Platform
Single-dose Chemiluminescense Immunoassay Platform Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.
Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously. Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.
Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals. Ready-to-use lyophilized RT-PCR Reagent;
Ready-to-use lyophilized RT-PCR Reagent; Wondfo PA-3600 IHC Staining System
Wondfo PA-3600 IHC Staining System The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds.
The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds. This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.
This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.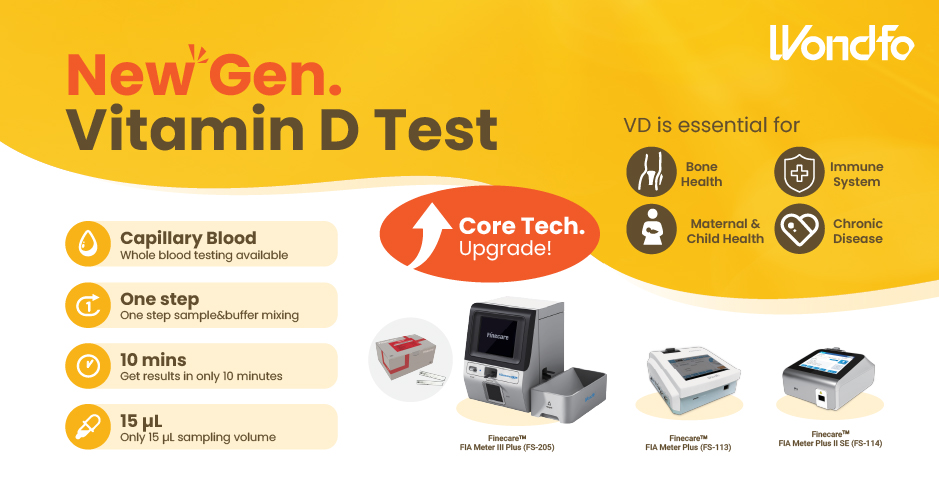 Finecare™ Vitamin D, from complexity to simplicity
Finecare™ Vitamin D, from complexity to simplicity Building A World Free from Antibitoic Overuse
Building A World Free from Antibitoic Overuse Advanced rapid diagnostic test with WHO prequalification for infectious disease
Advanced rapid diagnostic test with WHO prequalification for infectious disease The Future Intelligent Medical Assistant to Healthcare
The Future Intelligent Medical Assistant to Healthcare Fight against the pandemic through continuous innovation
Fight against the pandemic through continuous innovation