Recently, Wondfo has officially become a member of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), and will continue to enhance communication with international experts and actively participate in the research and development of international standards to contribute to the global standardization of laboratory medicine.

As a global organization of clinical chemistry and laboratory medicine, IFCC is committed to promoting the development of laboratory medicine, improving global healthcare services, and upgrading the level and quality of diagnosis and treatment by cooperating with other international organizations to develop global standards, organizing a series of professional and high-level forums, and supporting its members in their scientific and educational efforts to enhance the accuracy, reliability and comparability of laboratory tests. Since its establishment in 1952, IFCC has developed 119 national and regional members and 50,000+ expert members, making it one of the most influential organizations in the related fields worldwide.

Measurement Traceability Serves the World | Promotes Standardization and Consistency of Test Results
In order to promote the traceability of laboratory medicine measurement results, Wondfo has established the Reference Systems Department, which builds an uninterrupted chain of traceability from the System of International Units (SI)-Reference Laboratories-Clinical Laboratories to further promote the mutual recognition of test results.
Wondfo Qualifications
-Stakeholder members of the Joint Committee for Traceability in Laboratory Medicine (JCTLM)
-Chinese National Accreditation Service for Conformity Assessment (CNAS) laboratory accreditation qualifications
-12 items in the JCTLM Reference Measurement Services list
-IFCC-RELA 20 items all passed the test.

Becoming a member of IFCC will deepen the communication and cooperation between Wondfo and its international counterparts, provide timely access to the latest industry technical information, and enhance the laboratory's ability to trace back its measurements. Wondfo will also actively participate in the development and promotion of clinical chemistry and laboratory medicine standards, play a greater role in promoting the standardization and traceability of laboratory medicine measurements, and promote the mutual recognition of results globally.
Adhering to the vision of "striving to make biotechnology benefit all", Wondfo will continue to promote standardization and consistency, adhere to promote high-quality development with scientific research and innovation, so that enable IVD technology serve better the health for everyone.
 The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc.
The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc. 50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others.
50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others. Single-dose Chemiluminescense Immunoassay Platform
Single-dose Chemiluminescense Immunoassay Platform Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.
Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously. Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.
Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals. Ready-to-use lyophilized RT-PCR Reagent;
Ready-to-use lyophilized RT-PCR Reagent; Wondfo PA-3600 IHC Staining System
Wondfo PA-3600 IHC Staining System The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds.
The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds. This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.
This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.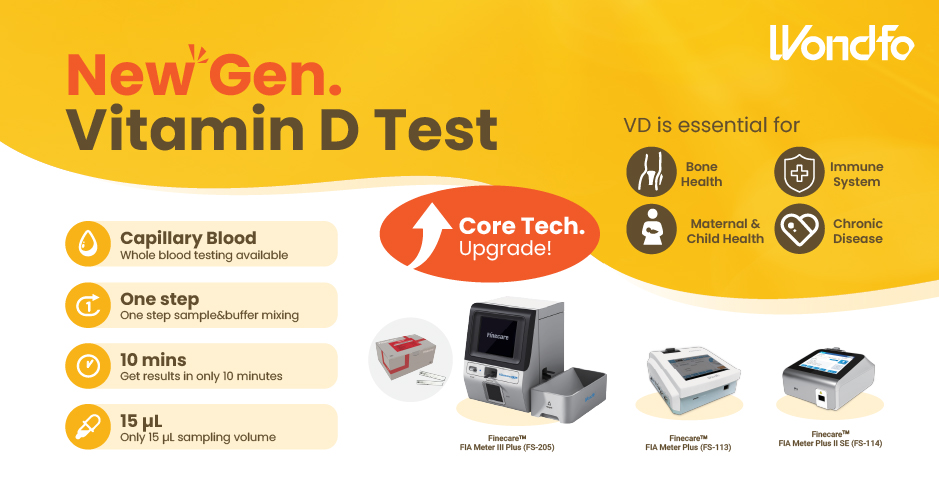 Finecare™ Vitamin D, from complexity to simplicity
Finecare™ Vitamin D, from complexity to simplicity Building A World Free from Antibitoic Overuse
Building A World Free from Antibitoic Overuse Advanced rapid diagnostic test with WHO prequalification for infectious disease
Advanced rapid diagnostic test with WHO prequalification for infectious disease The Future Intelligent Medical Assistant to Healthcare
The Future Intelligent Medical Assistant to Healthcare Fight against the pandemic through continuous innovation
Fight against the pandemic through continuous innovation