Recently, Wondfo's wholly-owned subsidiary in the United States, Wondfo USA Co., Ltd. (hereinafter referred to as the "Wondfo USA"), received notification from the U.S. Food and Drug Administration (FDA) that the Wondfo USA's WELLlife™ COVID-19 / Influenza A&B Home Test for the SARS-COV-2 viruses, influenza A virus, and influenza B virus, has obtained Emergency Use Authorization from FDA (EUA240011).
One Sample, Three Results, Easy and Fast
Respiratory virus infections caused by influenza A, influenza B, or the SARS-COV-2 viruses exhibit similar clinical manifestations. Based on lateral flow immunoassay, Wondfo USA’s WELLlife™ COVID-19 / Influenza A&B Home Test is easy to operate. Adding one sample, three results can be obtained in 10 minutes. It features a long detection window, built-in quality control line, and fast result show. Only an anterior nasal sample is needed for a qualitative testing within 5 days of symptom onset.
Based on the independent evaluation results by the FDA, third-party laboratory research, clinical trial, and usability study among the general population, Wondfo USA’s WELLlife™ COVID-19 / Influenza A&B Home Test has been proven to be safe, effective, simple to use, suitable for at-home testing in regular households, and appropriate for the use by individuals aged two and above. Clinical validation data shows that the test has a specificity of 99.7% compared to PCR results, and the sensitivity has far exceeded the FDA requirement of 80%.
In conclusion, the WELLlife™ COVID-19 / Influenza A&B Home Test features below advantages:
-
Suitable for testing respiratory patients aged two and above
-
Suitable for testing patients with respiratory symptoms within 5 days
-
Simple sampling with anterior nasal swab
-
With only one sample added, it can simultaneously test for the SARS-COV-2 viruses, influenza A, and influenza B
-
Results available in 10 minutes
-
Good product performance, with high clinical validation showing a high correlation between test results and laboratory testing
Building a Defense Line for Life with Technology
Fight Infectious Diseases together
To help early detection, diagnosis, and treatment of respiratory infectious diseases, Wondfo Biotech integrates multiple technology platforms, including colloidal gold, fluorescence immunoassay, chemiluminescence, electrochemistry, molecular diagnostics, etc., developing a one-stop respiratory infection testing solution from pathogen to serology, which can timely and effectively assist in the differential diagnosis and treatment of common respiratory diseases.
To rapidly identify infection types, Wondfo’s colloidal gold technology platform can provide rapid testing products for the SARS-COV-2 viruses and influenza A and B, mycoplasma/chlamydia pneumonia, etc., with simple operation and results available in 15 minutes.
For assessing the severity of infection, evaluating antibiotic efficacy, etc., Wondfo Biotech has achieved a leading coverage of inflammatory biomarkers domestically based on the dual platform of immunofluorescence and chemiluminescence, continuously iterated and upgraded to effectively assist in determining the severity of infection and prevent antibiotic misuse. Wondfo Biotech’s immunofluorescence strip enables fully automated micro blood testing of complete blood routine and four inflammation items with just one blood sample, requiring a minimum sample volume of only 8.5 microliters, providing quantitative results in 3-15 minutes. The chemiluminescence platform has high efficiency per unit area, good stability, precision, and repeatability, capable of detecting subtle concentration changes to aid in accurate diagnosis.
From HIV, SARS, influenza A and B, H7N9 to COVID-19, Wondfo has always been actively involved in global public health emergency responses, using technology to support epidemic prevention and control. Wondfo will continue promoting scientific research innovation, collaborating with global organizations to combat threats to human health posed by infectious diseases.
 The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc.
The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc. 50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others.
50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others. Single-dose Chemiluminescense Immunoassay Platform
Single-dose Chemiluminescense Immunoassay Platform Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.
Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously. Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.
Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals. Ready-to-use lyophilized RT-PCR Reagent;
Ready-to-use lyophilized RT-PCR Reagent; Wondfo PA-3600 IHC Staining System
Wondfo PA-3600 IHC Staining System The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds.
The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds. This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.
This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.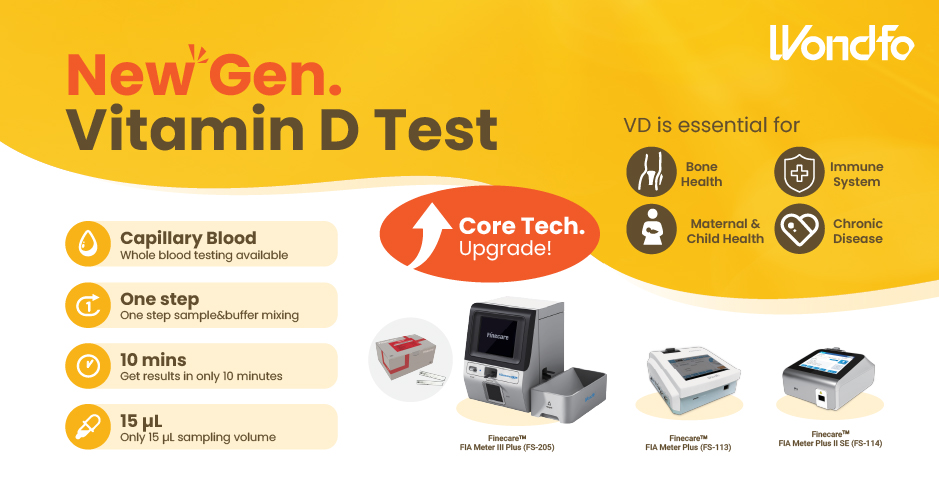 Finecare™ Vitamin D, from complexity to simplicity
Finecare™ Vitamin D, from complexity to simplicity Building A World Free from Antibitoic Overuse
Building A World Free from Antibitoic Overuse Advanced rapid diagnostic test with WHO prequalification for infectious disease
Advanced rapid diagnostic test with WHO prequalification for infectious disease The Future Intelligent Medical Assistant to Healthcare
The Future Intelligent Medical Assistant to Healthcare Fight against the pandemic through continuous innovation
Fight against the pandemic through continuous innovation