Recently, Clinical Chemistry and Laboratory Medicine, an authoritative journal of laboratory medicine with an impact factor of 8.490, published a research on the point-of-care detection of TSH (thyroid stimulating hormone) The essay verifies that Wondfo fluorescence immunoassay system can effectively assist in the clinical diagnosis of thyroid dysfunction. Since 2009 when Wondfo launched the first quantitative FIA analyzer independently developed domestically, 70,000+ Wondfo Finecare™ FIA Meters have been installed and applied in over 100 countries and regions worldwide.
Verified by internationally influential journal: Suitable for Clinical Use for the Diagnosis of Thyroid Dysfunction
As one of the leading journals in the field of laboratory medicine, Clinical Chemistry and Laboratory Medicine (hereinafter referred to as "CCLM") was established in 1963. Focusing on basic and applied research, and cutting-edge research in clinical laboratory medicine, CCLM enjoys an impact factor of 8.490. CCLM is the official journal of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM), as well as the official journal of the National Societies from Australia, Germany, Italy, Belgium, Slovenia, Spain, Portugal, Ireland and other countries.

In August this year, CCLM published an essay regarding measuring TSH—"A novel point-of-care device accurately measures thyrotropin in whole blood, capillary blood and serum”. The study was led by Prof. George J. Kahaly of the Molecular Thyroid Research Laboratory of the Gutenberg University (JGU) Medical Center in Mainz, Germany and Johannes Lotz of the JGU Institute for Clinical Chemistry and Laboratory Medicine. TSH measurements were made from 730 consecutive, unselected subjects in an outpatient setting, using Wondfo Finecare immunofluorescence assay, to assess its role in the timely diagnosis of thyroid function.

As TSH level reflects thyroid function, timely and accurate diagnosis is of great help for subsequent treatment for patients with suspected thyroid disease. Traditional laboratory testing for TSH usually takes two hours or more, while POCT can reduce turnaround time to minutes. To give full play to the advantages of POCT products, it is necessary to ensure that the performance of POCT products is consistent with that of traditional laboratory instruments. To this end, the researchers evaluated the performance of POCT in measuring TSH and compare it with an automated laboratory instrument.
Wondfo Finecare quantitative immunofluorescence assay provides ever more convenient rapid detection on site. Requiring no professional operation, no specific supporting instruments, and no cold chain storage and transportation, it enjoys simple operation and releases rapid result in 15 minutes. The research results show that Wondfo Finecare FIA Meter can effectively assist in the clinical diagnosis of thyroid dysfunction.
The First FIA System Developed Domestically, Breaking the Monopoly;
Leading in Installed Capacity in China with the Most Comprehensive Test Menu
As early as 2009, Wondfo launched the first quantitative fluorescence immunoassay detecting system independently developed domestically, with inflammation markers, cardiac markers and other test items gradually launched. It broke the long-term monopoly in the Chinese market. With the advantages of a comprehensive reagent panel, sample compatibility of whole blood/plasma/serum, and multi-item detection at one time, more than 20,000 units of Wondfo Finecare™ FIA Meters have been installed in China, ranking first in domestic installed capacity. Over 50,000 units have been installed in more than 100 countries and regions overseas, widely applied in various clinical scenarios such as laboratory, emergency, ICU, and cardiology.

At present, Finecare™ FIA Meters enable 50+ test items and cover a highly comprehensive series of categories, including cardiovascular markers, inflammatory markers, craniocerebral injury markers, diabetes and renal injury markers, tumor markers, thyroid hormone markers, etc. The types of reagents cover most complete. Relevant scientific research achievements won the second prize of the 2018 Guangdong Science and Technology Progress Award.
On February 25, 2022,
Merck, a globally leading technology company, and Wondfo signed a cooperation agreement, whereby both will leverage their respective strengths in thyroid disease treatment and in vitro diagnosis to increase the accessibility of thyroid function testing in community-level hospitals, and to help improve the capability of those hospitals in thyroid disease diagnosis and treatment.
As 2022 marks the 30
th anniversary of Wondfo, we will continue to be devoted to research and innovation in IVD industry. We remain unswerving to provide the public with cutting-edge technology and products and improve diagnosis with rapid tests. Racing for Life.
 The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc.
The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc. 50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others.
50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others. Single-dose Chemiluminescense Immunoassay Platform
Single-dose Chemiluminescense Immunoassay Platform Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.
Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously. Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.
Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals. Ready-to-use lyophilized RT-PCR Reagent;
Ready-to-use lyophilized RT-PCR Reagent; Wondfo PA-3600 IHC Staining System
Wondfo PA-3600 IHC Staining System The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds.
The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds. This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.
This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.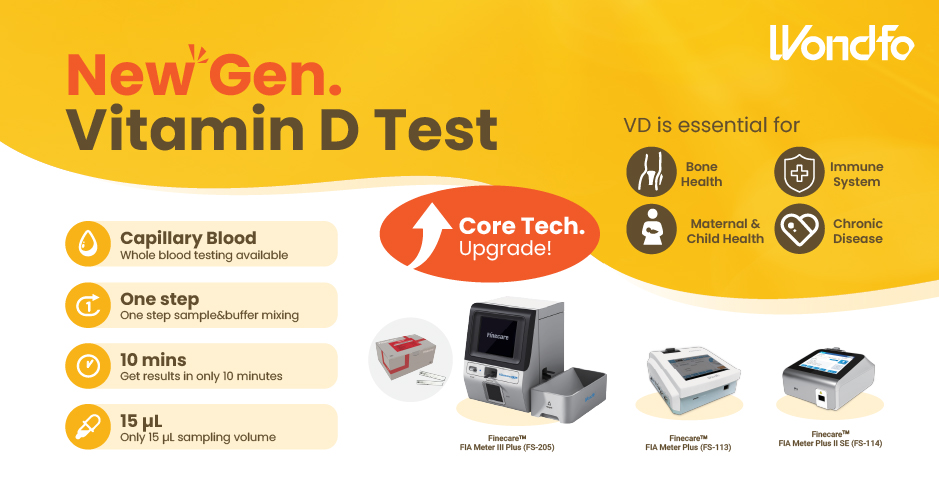 Finecare™ Vitamin D, from complexity to simplicity
Finecare™ Vitamin D, from complexity to simplicity Building A World Free from Antibitoic Overuse
Building A World Free from Antibitoic Overuse Advanced rapid diagnostic test with WHO prequalification for infectious disease
Advanced rapid diagnostic test with WHO prequalification for infectious disease The Future Intelligent Medical Assistant to Healthcare
The Future Intelligent Medical Assistant to Healthcare Fight against the pandemic through continuous innovation
Fight against the pandemic through continuous innovation