Clinical and Laboratory Standards Institute (CLSI) guidelines play a pivotal role in shaping laboratory practice. This article offers an accessible introduction to the CLSI guidelines on establishing reference intervals, focusing on a streamlined understanding of the fundamental principles and logic behind the process without delving into the intricacies of detailed statistical methods.
1. Introduction
Reference intervals are a fundamental aspect of clinical laboratory testing, providing a statistical framework for interpreting patient results. They are defined as the interval between two values, derived from a reference population, which typically includes the central 95% of the distribution of a measured analyte in a healthy population. These intervals are not fixed but rather are influenced by various factors, including age, sex, ethnicity, and physiological conditions.
Reference intervals serve as a comparative tool for clinicians to assess a patient's test results against a benchmark of 'normal' values. This comparison is crucial for diagnosing diseases, monitoring treatment progress, and making informed clinical decisions. Moreover, standardized reference intervals facilitate the sharing of laboratory data across different healthcare settings and geographical locations, promoting continuity of care and enhancing patient outcomes.
However, in practice, few laboratories conduct their own reference interval studies. Sometimes laboratories and manufacturers do not perform new reference interval studies, but refer to studies performed decades ago when the methods and populations were very different. Thus, we should also focus more on validating reference intervals.
2. Procedures for Establishing Reference Intervals
According to this guideline, establishing reference intervals begins with a thorough literature review to understand the analyte's biological variability and potential interferences. Following this, the selection of reference individuals is based on predefined exclusion and partitioning criteria, ensuring a representative and healthy sample group. The selection process involves comprehensive subject preparation, including questionnaires and health assessments, to categorize and exclude individuals who do not meet the health criteria. The selected reference individuals are then subjected to specimen collection, which must be performed under standardized conditions to ensure consistency. The specimens are analyzed using validated analytical methods, and the resulting data is scrutinized for outliers and distribution patterns. Then the reference limits and intervals will be calculated by a selected estimation method. At the end, all the aforementioned steps and procedures should be documented thoroughly.
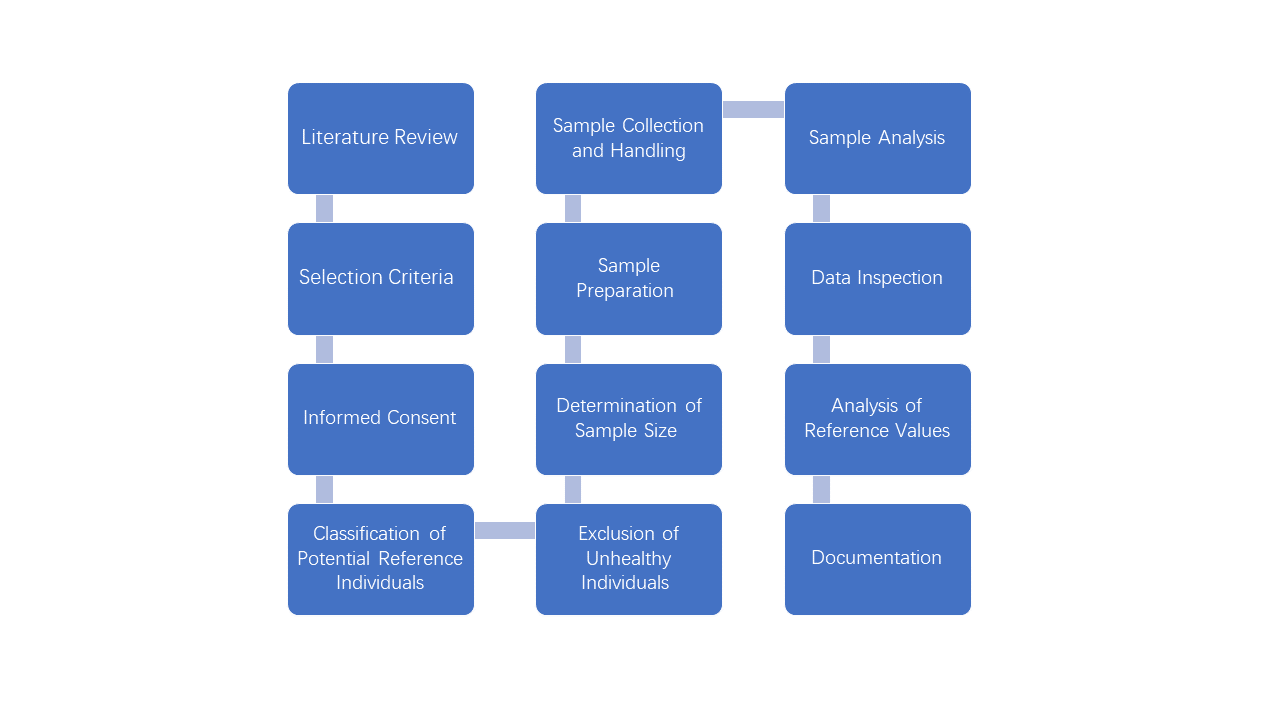
Figure-1 Procedures for Establishing Reference Intervals
3. Application of Statistical Methods in Determining Reference Intervals
The applications of both nonparametric and parametric statistical methods for the estimation of reference intervals were discussed in the CLSI guideline.
The nonparametric method is the preferred method for estimating reference intervals due to its simplicity, reliability, and lack of assumptions about the data distribution.
The simple nonparametric method remains the recommended procedure for establishing reference intervals if a laboratory has limited access to statistical and computational support. This guideline proposed nonparametric method as the preferred method for analysis. However, it may be less efficient with smaller sample sizes.
In contrast, the parametric method, which assumes a normal distribution of data, often requires data transformation to meet the assumption of normality, followed by the estimation of reference limits using statistical models. While the parametric method can provide more precise estimates with smaller sample sizes but is dependent on the normality assumption, which may not always hold true, particularly for skewed data distributions.
4. Verification and Transfer of Reference Intervals
The determination of reliable reference intervals can be a major and costly task. As more new tests and methods are introduced in laboratories, it is unrealistic to expect each laboratory to develop its own reference intervals independently. Therefore, it is very useful to be able to transfer a reference interval from one laboratory to another by some process less costly and more convenient. The CLSI working group believes that verifying reference intervals established elsewhere (e.g., manufacturers' product inserts) is feasible for most individual laboratories.
4.1 Verification of Reference Intervals
Verification is the process of ensuring that a reference interval, established by another laboratory or from a different population, is applicable to the local context. This process typically involves analyzing a small number of samples from a local reference population and comparing the results against the established reference interval. Verification is crucial for adapting reference intervals to local populations with unique characteristics or for confirming the suitability of transferred intervals.
4.2 Transfer of Reference Intervals
Transferring reference intervals from one analytical system or population to another is a practical approach to utilizing existing data. This process requires careful consideration of the comparability of the analytical methods and the similarity of the test subject populations. The guideline provides statistical methods and protocols for assessing the comparability and suitability of transferred reference intervals, ensuring their validity and reliability for the intended use.
4.3 The Applicable Situations of Three Approaches of the Validation of Reference Interval Transference
(1) Subjective assessment
If, in the judgment of the laboratorian, these factors are consistent with the receiving laboratory's operation and test subject population, then the reference interval maybe transferred without a requirement for any receiving laboratory validation studies, other than a documentation of these considerations.
(2) Using small number of reference individuals
A user or receiving laboratory may wish to, or maybe required to, validate the transference of a reference interval reported by a manufacturer or other donor laboratory. If there are substantial differences in the geographic locations or demographic variables of the two populations that are known to cause differences in the reference values, there is little point in trying to transfer the reference interval.
(3) Using large number of reference individuals
Laboratories may elect to undertake a more extensive reference interval transference study for analytes whose reference intervals are critically important for local clinical interpretation of the assay.
5. Practicality of the Guideline and Future Development
This CLSI guideline points out the challenges faced by clinical laboratories in establishing reference intervals. And it offers pragmatic solutions, such as the verification and transfer of reference intervals, which can be more feasible for many laboratories than conducting a full-scale reference interval study. The guideline also encourages the use of modern statistical techniques and the participation of laboratories in multicenter studies to leverage shared data and reduce the individual burden of reference interval establishment.
Technological advancements in laboratory testing, data analysis, and information systems are expected to have a profound impact on the field of reference intervals. Advanced bioinformatics tools and personalized medicine approaches may lead to a more detailed understanding of individual variability and the development of individualized reference ranges. Furthermore, the increasing use of electronic health records and data sharing platforms could facilitate the collection and analysis of large, diverse datasets, enhancing the representativeness and applicability of reference intervals across diverse populations.
Reference
Horowitz, G. L., Altaie, S., & Boyd, J. C. (2010). Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline. CLSI.
 The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc.
The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc. 50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others.
50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others. Single-dose Chemiluminescense Immunoassay Platform
Single-dose Chemiluminescense Immunoassay Platform Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.
Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously. Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.
Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals. Ready-to-use lyophilized RT-PCR Reagent;
Ready-to-use lyophilized RT-PCR Reagent; Wondfo PA-3600 IHC Staining System
Wondfo PA-3600 IHC Staining System The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds.
The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds. This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.
This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers. Finecare™ Vitamin D, from complexity to simplicity
Finecare™ Vitamin D, from complexity to simplicity Building A World Free from Antibitoic Overuse
Building A World Free from Antibitoic Overuse Advanced rapid diagnostic test with WHO prequalification for infectious disease
Advanced rapid diagnostic test with WHO prequalification for infectious disease The Future Intelligent Medical Assistant to Healthcare
The Future Intelligent Medical Assistant to Healthcare Fight against the pandemic through continuous innovation
Fight against the pandemic through continuous innovation