Cardiac troponin (cTn) testing plays a vital role in the early diagnosis, risk stratification, monitoring, and management of acute myocardial infarction (AMI). POC devices are crucial in delivering timely and effective patient care, especially in acute settings such as acute chest pain attack.
Benefits of POC cTn tests
1. Reduced TAT for Emergency Patients
POC testing can reduce the overall turnaround time (time from blood draw to result) for results by reducing the time needed for sample transport and processing, reportedly saving 18-93 mins if comparing with central lab test [1]. POC tests facilitates the rapid transmission of results directly to clinicians. This expedited process enables healthcare providers to promptly assess patient status, leading to immediate interventions that positively impact patient outcomes.
2. Improved Patient Flow
By offering a faster diagnostic process, POC testing can help optimize patient flow through the emergency department, potentially reducing the length of stay (reduce 0.2-2.7 hours) in the emergency department and improving the efficiency of emergency medical services [2].
3. Accessibility
POC testing can be particularly beneficial in settings where central laboratory services are not available 24/7, such as in smaller hospitals or remote areas, providing a means for rapid assessment and disposition of patients [3].
Precautions when using POC cTn assay [4]
a) The relationship between POC cTn & Central lab cTn
POCT instrument could be an attractive and cost-effective possibility for healthcare professionals. However, we should be aware of its relationship with central lab cTn. In the institution without central lab, the POC can be a good alternative to provide value information. While in the institution with central lab, the relationship between POC and central lab need to be noted:
1. The POC and central laboratory assays are two different cTn assays for which 99th percentiles and delta values used in algorithms may significantly differ.
2. If a patient sample has a cTn level above 99th percentiles by POC testing, the very same sample should be analyzed by the central lab cTn assay if the patient is admitted. The only exception is if repeat testing is always by POC testing.
3. Diagnoses using serial samplings must only be based on one assay. POC and central lab results typically cannot be used interchangeably.
4. Monitor the quality of POC cTn assay over time with central lab. The central lab can be alerted if the results start to deviate by continuously comparing these two results.
b) Sample type
1. It is not recommended to use venous and capillary samples interchangeably to trend values.
2. Discordance between results can be established by paired analysis of consecutive blood samples on the central lab cTn assay (plasma and serum) and the POC whole blood cTn assay by central lab personnel.
c) Quality control
The POC system should include regular automatic system checks or there should be a regular maintenance procedure to verify instrument performance.
Conclusions
POC cTn assays provide swift diagnoses, enhancing emergency department efficiency. They are especially valuable in facilities with limited lab access. A clear and rational clinical workflow for POC cTn assay is conducive to maximizing its effectiveness.
Exploring Perspectives: Do you support the use of POC cTn assay in emergency medicine?
Reference:
[1] https://www.ncbi.nlm.nih.gov/books/NBK362844/
[2] Loten, Conrad et al. “Point of care troponin decreases time in the emergency department for patients with possible acute coronary syndrome: a randomised controlled trial.” Emergency medicine journal : EMJ vol. 27,3 (2010): 194-8. doi:10.1136/emj.2008.069427
[3] Thulin, Ingrid Viola Lavesson, et al. "Point-of-care high-sensitivity troponin assays: Advancements, clinical applications, and implementation considerations." (2023)
[4] Collinson et al. "Cardiac troponin measurement at the point of care: educational recommendations on analytical and clinical aspects by the IFCC Committee on Clinical Applications of Cardiac Bio-Markers (IFCC C-CB)" Clinical Chemistry and Laboratory Medicine (CCLM), vol. 61, no. 6, 2023, pp. 989-998. https://doi.org/10.1515/cclm-2022-1270
Wondfo producs
Chemiluminescence Immunoassay
A8
A90
Immunofluorescence Assay
FS-2000
FS-1000
FS-113
FS-114
 The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc.
The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc. 50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others.
50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others. Single-dose Chemiluminescense Immunoassay Platform
Single-dose Chemiluminescense Immunoassay Platform Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.
Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously. Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.
Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals. Ready-to-use lyophilized RT-PCR Reagent;
Ready-to-use lyophilized RT-PCR Reagent; Wondfo PA-3600 IHC Staining System
Wondfo PA-3600 IHC Staining System The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds.
The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds. This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.
This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.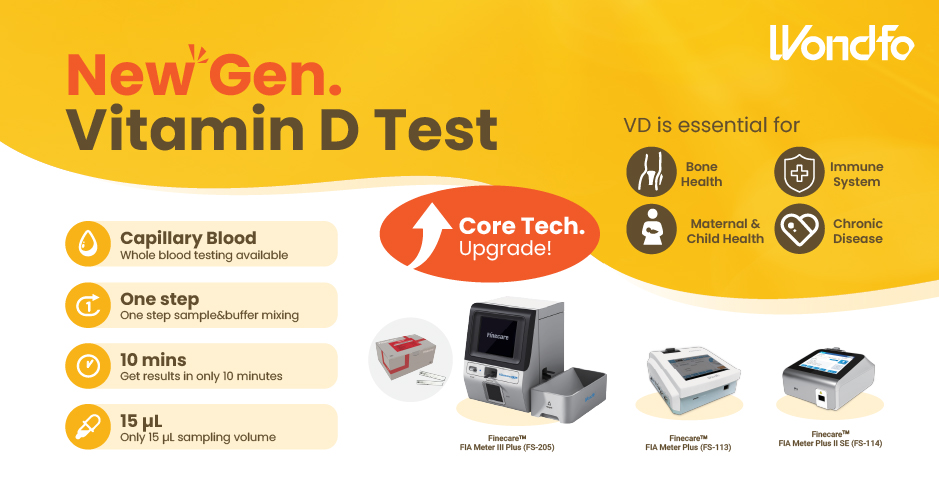 Finecare™ Vitamin D, from complexity to simplicity
Finecare™ Vitamin D, from complexity to simplicity Building A World Free from Antibitoic Overuse
Building A World Free from Antibitoic Overuse Advanced rapid diagnostic test with WHO prequalification for infectious disease
Advanced rapid diagnostic test with WHO prequalification for infectious disease The Future Intelligent Medical Assistant to Healthcare
The Future Intelligent Medical Assistant to Healthcare Fight against the pandemic through continuous innovation
Fight against the pandemic through continuous innovation