As an acute and critical disease, sepsis has a high morbidity and mortality rate, with approximately 48.9 million sepsis cases recorded globally in 2017, and nearly 1 in 5 deaths attributed to sepsis [1]. WHO has identified sepsis as a major public health problem. A recent study published in the journal Diagnostics shows that Wondfo’s quantitative Fluorescence Detection System can provide powerful support for early detection and intervention of sepsis. Integrating multiple platforms and product lines, Wondfo can provide effective support for early detection of sepsis, disease monitoring, medication guidance, and efficacy assessment.

Reliable POC Testing is Critical to Reduce Mortality
A research team from the University of Technology Malaysia (UTM), one of the top 1% universities in the world, conducted a study on critically ill sepsis patients in ICUs to assess the concordance between PCT blood results obtained by POC testing and traditional biochemical immunoassay systems, and the results were published in May 2024 in the journal Diagnostics.
The research team noted that the key to the diagnosis and treatment of sepsis is early detection and intervention. Sending samples to a central laboratory for testing is complicated and has a long turnaround time, so there can be delays in reporting PCT results. Therefore, rapid and accurate results using a high-quality POC assay are essential to assist clinicians in making timely decisions and earning more time for sepsis patients.
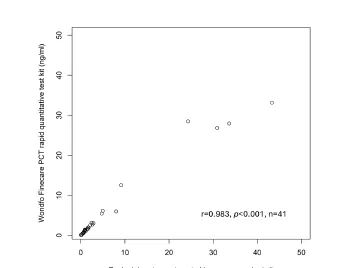
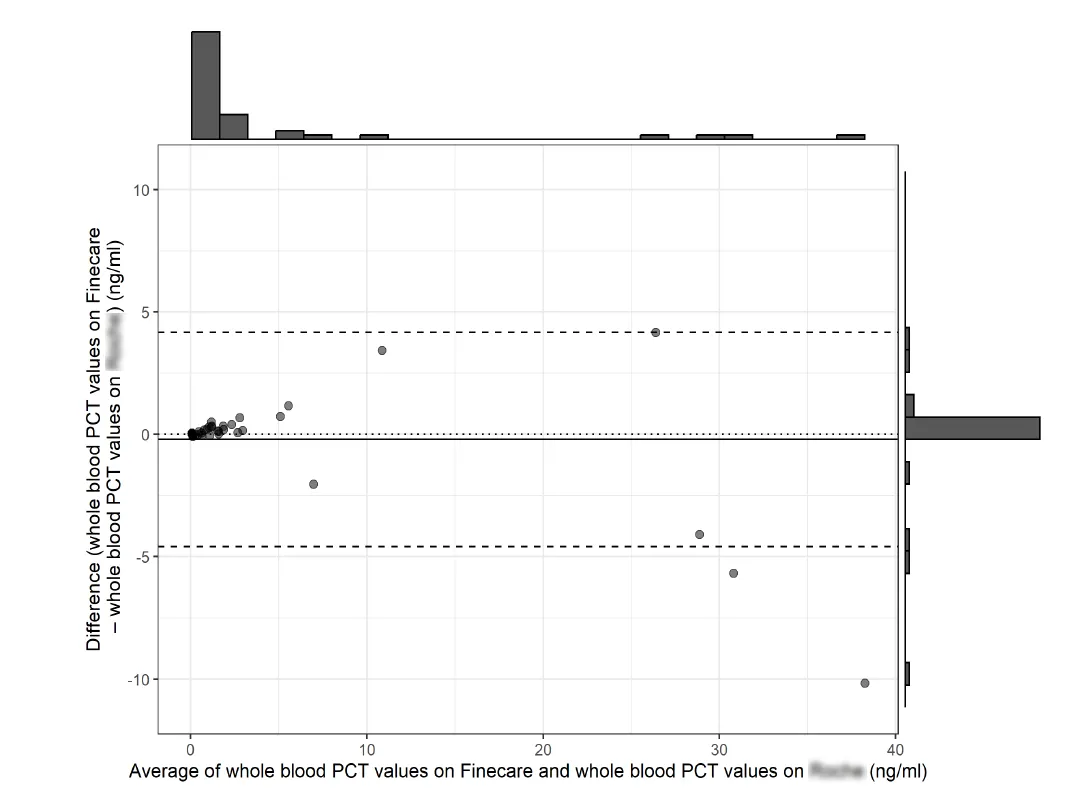
In this study, blood samples were collected from 41 critically ill sepsis patients, and after centrifuging the whole blood samples into plasma samples, PCT measurements were performed using Wondfo’s quantitative Fluorescence Detection System and the world's leading biochemical immunoassay system, respectively. The results showed a significant correlation between the results of the two assays, with a sample correlation coefficient of 0.98. Additionally, the study showed good precision with CV values of 5% and 2.5% at 0.5ng/mL and 0.25ng/mL, respectively.
In the conclusion, the research team pointed out that Wondfo’s quantitative Fluorescence Detection System can provide a more rapid and affordable PCT assay, which can provide a reliable and robust support for the monitoring of the relevant indexes of critically ill sepsis patients in ICUs [2].
Full series + The first in China to provide effective assistance for the whole process of sepsis testing
According to the “China Expert Consensus on Early Prevention and Interruption of Sepsis in Emergency Care”, timely identification of patients with acute infections and provision of reasonable treatment is the first step in sepsis prevention. Currently, commonly used biochemical markers of infection include CRP, PCT, IL-6, SAA and HBP [3]. Studies have shown that elevated levels of TM, TAT, PIC, and t-PAIC play an important role in determining the severity of sepsis patients [4-5].
Responding to the health needs of the public, Wondfo has developed a full series of infection marker products around immunofluorescence platform and chemiluminescence platform, which can achieve the diagnosis of different combinations of IL-6, CRP, PCT, SAA, and hs-CRP, and launched the first domestic thrombosis early screening program – Thrombosis 4-Item Test (TM, TAT, PIC, t-PAIC), which can effectively help identify the type of sepsis infection and play an important role in the early detection of sepsis, monitoring of the condition, guidance of medication and evaluation of the efficacy of treatment.
As the first product in China to achieve the simultaneous detection of six thrombus items with one machine and one tube of blood, Wondfo’s chemiluminescence Thrombosis 4-Item Test has passed the external quality assessment with excellent performance in the 2022 Beijing-Tianjin-Hebei-Shandong Joint external quality assessment activity. This is also the first time for a domestic thrombus program to pass the external quality assessment, which fully proves the accuracy and reliability of the results of Wondfo’s Thrombosis 4-Item Test.
Wondfo has always been dedicated to the health of all people, striving for perfection and excellence. We continuously improve the diagnostic efficiency of medical institutions, safeguarding life and health with innovative achievements. Our vision is to make biotechnology benefit all.
References
[1]王伊帆, 陈燕, 彭劲民, 杜斌, 翁利. 中国脓毒症流行病学的研究进展[J]. 中华重症医学电子杂志, 2023, 09(01): 89-94.
[2] Mazlan, .Z.; Wan Azman, W.N.; Yaacob, N.M.; Koon, T.S.; Yahya, N.K. Analytical Evaluation of Point-of-Care Finecare™ Procalcitonin Rapid Quantitative Test in Sepsis Population as Compared with Elecsys® BRAHMS Procalcitonin Immunoassay. Diagnostics 2024, 14, 1080. https://doi.org/10.3390/diagnostics14111080
[3]中国医药教育协会感染疾病专业委员会.感染相关生物标志物临床意义解读专家共识[J].中华结核和呼吸杂志,2017,40(4):243-257.
[4]林静,孙志鹏,李娟,等. 联合检测TM、TAT、PIC、t-PAIC水平对DIC的诊断价值[J]. 国际检验医学杂志,2019,40(12):1413-1416. DOI:10.3969/j.issn.1673-4130.2019.12.002.
[5]曾镇桦,高海闽,黄绍生,等. 脓毒症患者TAT、TM、PIC和t-PAIC水平与病情程度的关系[J]. 疑难病杂志,2021,20(12):1240-1243. DOI:10.3969/j.issn.1671-6450.2021.12.012.
 The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc.
The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc. 50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others.
50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others. Single-dose Chemiluminescense Immunoassay Platform
Single-dose Chemiluminescense Immunoassay Platform Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.
Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously. Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.
Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals. Ready-to-use lyophilized RT-PCR Reagent;
Ready-to-use lyophilized RT-PCR Reagent; Wondfo PA-3600 IHC Staining System
Wondfo PA-3600 IHC Staining System The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds.
The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds. This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.
This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers. Finecare™ Vitamin D, from complexity to simplicity
Finecare™ Vitamin D, from complexity to simplicity Building A World Free from Antibitoic Overuse
Building A World Free from Antibitoic Overuse Advanced rapid diagnostic test with WHO prequalification for infectious disease
Advanced rapid diagnostic test with WHO prequalification for infectious disease The Future Intelligent Medical Assistant to Healthcare
The Future Intelligent Medical Assistant to Healthcare Fight against the pandemic through continuous innovation
Fight against the pandemic through continuous innovation