July 13
th, 2022, Wondfo HIV Self-Test is pre-qualified by the World Health Organization (WHO), which marks a milestone for Wondfo to help improving accessibility and availability of rapid diagnosis to infectious diseases worldwide especially in low-income countries.
WHO prequalification seeks to ensure a supply of quality-assured products—medicines, vaccines, in vitro diagnostics, other medical devices particularly for use in low-income countries. With the endorsement of WHO prequalification, Wondfo HIV Self-Test can be accessible in most low-income countries which fit into the global health management strategy funded by major donors like Global Fund, PEPFAR, Clinton Health Access Initiative etc.
Wondfo’s Journey to WHO Prequalification
Wondfo developed independently One Step HIV 1/2 Whole Blood/Serum/Plasma Test (for professional use) in 2002 and it was prequalified by WHO in 2018. The effort to the long journey reveals our intention to reach a higher standard and promote products worldwide.
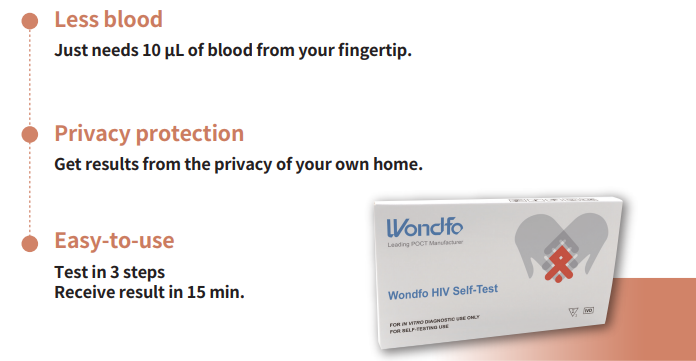
Wondfo HIV Self-Test can be done with fingerstick blood for HIV detection at home. simple testing procedure, minimal specimen volume (10 μL fingertip blood) and 15 mins to get the result.
As 2022 marks the 30
th anniversary of Wondfo, we will continue to be devoted to research and innovation in IVD industry. With varied and improved products and services, we assist in HIV control worldwide, to jointly achieve the goal of ending HIV/AIDS by 2030.
 The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc.
The first developed technology platform with various application scenarios, including infectious disease, fertility, DOA, etc. 50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others.
50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others. Single-dose Chemiluminescense Immunoassay Platform
Single-dose Chemiluminescense Immunoassay Platform Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.
Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously. Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.
Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals. Ready-to-use lyophilized RT-PCR Reagent;
Ready-to-use lyophilized RT-PCR Reagent; Wondfo PA-3600 IHC Staining System
Wondfo PA-3600 IHC Staining System The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds.
The Wondfo Truth-H80E HPLC Hemoglobin Analyzer is a high-performance diagnostic device designed for rapid and precise measurement of glycosylated hemoglobin (HbA1c)—the gold standard for diabetes diagnosis.It support the standard mode and variant mode and provide the result within 60-90seconds. This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.
This year, the summit will place a special focus on collaboration across different fields to explore the application of POCT and optimize clinical pathways. In the meantime, drive innovation by adoption of new technologies and biomarkers.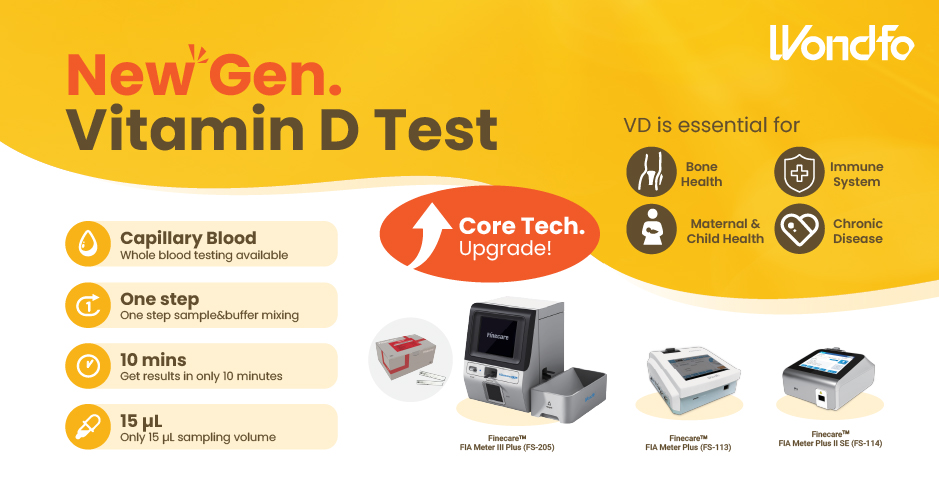 Finecare™ Vitamin D, from complexity to simplicity
Finecare™ Vitamin D, from complexity to simplicity Building A World Free from Antibitoic Overuse
Building A World Free from Antibitoic Overuse Advanced rapid diagnostic test with WHO prequalification for infectious disease
Advanced rapid diagnostic test with WHO prequalification for infectious disease The Future Intelligent Medical Assistant to Healthcare
The Future Intelligent Medical Assistant to Healthcare Fight against the pandemic through continuous innovation
Fight against the pandemic through continuous innovation